钯-单电子转移催化1-萘酰胺的C4远端胺化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
实现多环芳烃的位点选择性远端C-H胺化是一个具有挑战性但令人兴奋的目标。在此,我们报道了通过单电子转移策略对缺电子的1-萘酰胺进行远程C4胺化。该方案具有良好的官能团相容性,不需要螯合助剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
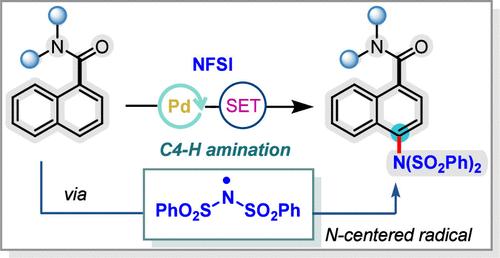
Site-Selective Remote C4 Amination of 1-Naphthamides via Palladium–Single-Electron Transfer Catalysis
Achieving site-selective remote C–H amination of polycyclic aromatic hydrocarbons is a challenging yet exciting goal. Herein, we report the remote C4 amination of electron-deficient 1-naphthamides through a single-electron transfer strategy. This protocol features excellent functional group compatibility and does not require a chelating auxiliary.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


