IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
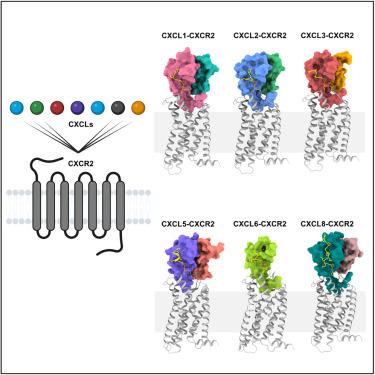
天然激动剂对其同源受体的选择性是 G 蛋白偶联受体(GPCR)的一大特点;然而,这种选择性在趋化因子受体上却经常被打破。趋化因子通常会与趋化因子受体发生杂乱的结合,但其潜在的分子决定因素大多仍然难以捉摸。在这里,我们对所有已知的 C-X-C 型趋化因子与每种 C-X-C 型趋化因子受体进行了全面的转导偶联分析,以生成该系统内编码的选择性和杂合性的全球指纹。在此基础上,我们确定了杂合性最强的受体--C-X-C 趋化因子受体 2(CXCR2)与几种趋化因子复合物的冷冻电镜(cryo-EM)结构。这些结构快照阐明了配体-受体相互作用的细节,包括结构基团,并通过诱变和功能实验进行了验证。我们还观察到,大多数趋化因子在 CXCR2 上的定位是二聚体,而 CXCL6 则表现出单体结合的姿态。总之,我们的研究结果为趋化因子在 CXCR2 上的杂合性提供了分子基础,对开发治疗分子具有潜在的意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular basis of promiscuous chemokine binding and structural mimicry at the C-X-C chemokine receptor, CXCR2
Selectivity of natural agonists for their cognate receptors is a hallmark of G-protein-coupled receptors (GPCRs); however, this selectivity often breaks down at the chemokine receptors. Chemokines often display promiscuous binding to chemokine receptors, but the underlying molecular determinants remain mostly elusive. Here, we perform a comprehensive transducer-coupling analysis, testing all known C-X-C chemokines on every C-X-C type chemokine receptor to generate a global fingerprint of the selectivity and promiscuity encoded within this system. Taking lead from this, we determine cryoelectron microscopy (cryo-EM) structures of the most promiscuous receptor, C-X-C chemokine receptor 2 (CXCR2), in complex with several chemokines. These structural snapshots elucidate the details of ligand-receptor interactions, including structural motifs, which are validated using mutagenesis and functional experiments. We also observe that most chemokines position themselves on CXCR2 as a dimer while CXCL6 exhibits a monomeric binding pose. Taken together, our findings provide the molecular basis of chemokine promiscuity at CXCR2 with potential implications for developing therapeutic molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


