基于PML::RARα基因组结构的急性早幼粒细胞白血病独特的白血病发生机制
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
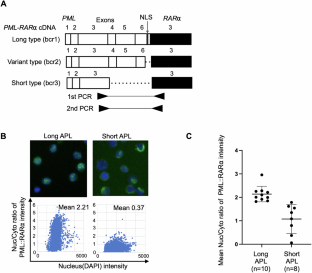
急性髓性白血病(AML)的白血病干细胞(LSCs)可以在CD34+CD38-片段中富集,并在体内重建人类AML。然而,急性早幼粒细胞白血病(APL)占所有AML病例的10%,由早幼粒细胞白血病-视黄酸受体α (PML::RARα)融合基因驱动,由于难以在体内有效重建人类APL, LSCs的存在长期未被确定。本文中,我们发现短型同种型APL(由PML基因的不同断点定义的APL亚型)的LSCs集中在CD34+CD38−部分并表达T细胞免疫球蛋白粘蛋白-3 (TIM-3)。与其他类型的APL相比,短型APL细胞表现出不同的基因表达特征,包括lsc相关基因。此外,CD34+CD38−TIM-3+短型APL细胞在高渗透的异种移植模型中有效地重建了人类APL,而CD34−分化的APL细胞则没有。此外,CD34+CD38−TIM-3+短型APL细胞在连续移植后重建白血病细胞。因此,短型APL是由自我更新的APL- lscs分层组织的。在APL亚群中鉴定LSCs并建立有效的患者来源的异种移植模型可能有助于进一步了解APL白血病的发生,并设计出根除APL LSCs的个性化治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Distinct leukemogenic mechanism of acute promyelocytic leukemia based on genomic structure of PML::RARα
Leukemic stem cells (LSCs) of acute myeloid leukemia (AML) can be enriched in the CD34+CD38- fraction and reconstitute human AML in vivo. However, in acute promyelocytic leukemia (APL), which constitutes 10% of all AML cases and is driven by promyelocytic leukemia-retinoic acid receptor alpha (PML::RARα) fusion genes, the presence of LSCs has long been unidentified because of the difficulty in efficient reconstitution of human APL in vivo. Herein, we show that LSCs of the short-type isoform APL, a subtype of APL defined by different breakpoints of the PML gene, concentrate in the CD34+CD38− fraction and express T cell immunoglobulin mucin-3 (TIM-3). Short-type APL cells exhibited distinct gene expression signatures, including LSC-related genes, compared to the other types of APL. Moreover, CD34+CD38−TIM-3+ short-type APL cells efficiently reconstituted human APL in xenograft models with high penetration, whereas CD34− differentiated APL cells did not. Furthermore, CD34+CD38−TIM-3+ short-type APL cells reconstituted leukemia cells after serial transplantation. Thus, short-type APL was hierarchically organized by self-renewing APL-LSCs. The identification of LSCs in a subset of APL and establishment of an efficient patient-derived xenograft model may contribute to further understanding the APL leukemogenesis and devise individual treatments for the eradication of APL LSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


