用意想不到的方法轻松合成 Housanes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
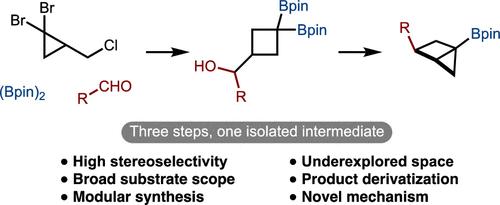
Facile Synthesis of Housanes by an Unexpected Strategy
Rigid bicyclic hydrocarbons have emerged as important building blocks in the drug discovery industry. Despite progress in this general area, bicyclo[2.1.0]pentanes (housanes) are an understudied class of molecules. Herein we report an unconventional synthesis of borylated housanes. Our method features a broad scope and high diastereoselectivities in the synthesis of versatile intermediates. The route involves a strain-release diboration of bicyclo[1.1.0]butane and intramolecular deborylative alkylation. The versatility of the bridgehead boronic ester was demonstrated in several functionalizations. Lastly, the mechanism of the reaction was investigated, and an unusual stereospecific and diastereoselective ring expansion was uncovered.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


