在中断的Ugi和Passerini反应中导航未开发的领域
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
中断的反应改变了既定的过程,常常导致意想不到的和新颖的结果。通过使用一个包含酸性和氧官能团的构建块,我们发现了Ugi和Passerini反应的中断变体。已经合成了20多个具有肽样框架的衍生物,表明了这些反应的广泛范围和多功能性。进一步的研究探索了使用各种亲核试剂和后修饰来扩大更多的化学多样性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
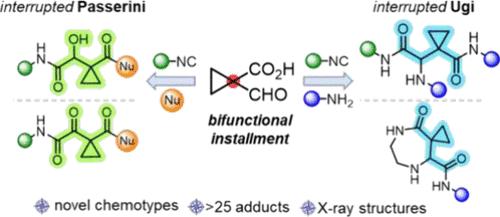
Navigating Unexplored Territories of the Interrupted Ugi and Passerini Reactions toward Peptidomimetics
Interrupted reactions redirect established processes, often resulting in unexpected and novel outcomes. By employing a building block containing both acidic and oxo functionalities tethered to the same carbon, we uncovered interrupted variants of the Ugi and Passerini reactions. More than 20 derivatives with a peptide-like framework have been synthesized, demonstrating the broad scope and versatility of these reactions. Additional studies explored the use of various nucleophiles and postmodifications to expand even more the chemical diversity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


