可见光辐照下可回收 g-C3N4 催化 N-芳基甘氨酸与乙烯基砜的脱羧烯化反应
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d5qo00100e
引用次数: 0
摘要
烯丙胺是一种用途广泛的化合物,在药物和有机合成中具有重要的应用。在此,我们提出了一种直接的方案,即可见光驱动g- c3n4催化N -芳基甘氨酸与乙烯基砜脱羧烷基化,以中等至优异的产率(高达91%)获得烯丙胺,该方案证明了广泛的底物兼容性,包括初级,二级,叔氨基甘氨酸和各种乙烯基砜。值得注意的是,g-C3N4催化剂可循环使用多达5次,而没有明显的催化性能损失。初步的机理研究表明,可见光是实现有效转换的必要条件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
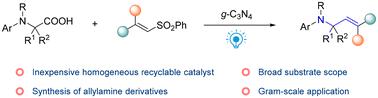
Recyclable g-C3N4 catalyzed decarboxylative alkenylation of N-aryl glycines with vinyl sulfones under visible-light irradiation†
Allylamines are a versatile class of compounds with significant applications in pharmaceuticals and as building blocks in organic synthesis. Herein we present a straightforward protocol for visible-light driven g-C3N4-catalyzed decarboxylative alkenylation of N-aryl glycines with vinyl sulfones to access allylamines in moderate to excellent yields (up to 91%), and it demonstrated broad substrate compatibility, including primary, secondary, and tertiary N-aryl glycines and diverse vinyl sulfones. Notably, the g-C3N4 catalyst is recyclable up to five times without obvious loss of catalytic performance. Preliminary mechanistic studies indicated that visible light is essential to achieve the desired transformation efficiently.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


