摘要
在呼吸系统疾病期间,肺巨噬细胞的数量、比例和极化都会发生动态变化。监测这些变化对于了解它们在病理学中的作用、改善诊断和指导药物开发至关重要。然而,目前基于组织活检的分析方法具有侵入性和静态性,限制了其提供此类动态信息的能力。在此,我们报告了一种双锁定巨噬细胞特异性肾脏可清除探针(DMRPNOCas),用于在甲型流感病毒(IAV)感染期间动态监测肺巨噬细胞。DMRPNOCas 在两种生物标记物(caspase-1 和 NO)(仅由 M1 巨噬细胞共同表达)存在的情况下激活荧光。为了优化 NO 反应活性,DMRPNOCas 的支架是从吲哚环上邻苯二胺基团位置不同的半氰基衍生物中筛选出来的。值得注意的是,与元取代的邻苯二胺相比,对位取代的邻苯二胺显示出更高的 NO 激活荧光。量子化学计算显示,这种增强归因于对 NO 反应引起的分子内电荷转移扭曲的不同抑制。DMRPNOCas 能将 M1 巨噬细胞与其他白细胞(包括 T 细胞、中性粒细胞和 M2 巨噬细胞)区分开来,这是单锁对照探针和其他已报道探针所无法比拟的。因此,DMRPNOCas 能对肺巨噬细胞进行体内动态监测,发现在 IAV 感染 48 小时内,单核细胞衍生的巨噬细胞广泛招募并出现 M1 极化。这一过程伴随着肺泡巨噬细胞的显著减少。这些发现为巨噬细胞介导的肺部炎症提供了新的见解,并强调了双锁定探针在精确诊断和监测病理过程方面的潜力。
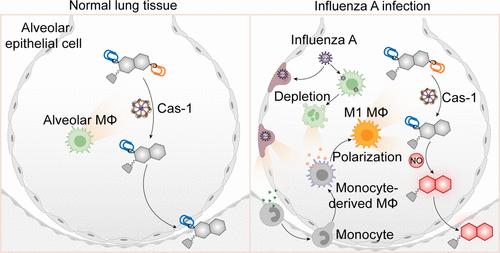
Pulmonary macrophages undergo dynamic changes in population, proportion, and polarization during respiratory diseases. Monitoring these changes is critical for understanding their roles in pathology, improving the diagnosis, and guiding drug development. However, current analytic methods based on tissue biopsy are invasive and static, limiting their ability to provide such dynamic information. Herein, we report a dual-locked macrophage-specific renal-clearable probe (DMRPNOCas) for the dynamic monitoring of pulmonary macrophages during influenza A virus (IAV) infection. DMRPNOCas activates fluorescence in the presence of two biomarkers (caspase-1 and NO) only coexpressed by M1 macrophages. To optimize the NO reactivity, the scaffold of DMRPNOCas is screened from the hemicyanine derivatives with an o-phenylenediamine group positioned differently on the indole ring. Notably, the para-substituted o-phenylenediamine demonstrates a higher NO-activated fluorescence compared to its meta-substituted counterpart. This enhancement, as revealed by quantum chemical calculations, is attributed to differential inhibition of twisted intramolecular charge transfer induced by the NO reaction. DMRPNOCas specifically distinguishes M1 macrophages from other leukocytes including T cells, neutrophils, and M2 macrophages, a capability unmatched by single-locked control probes and other reported probes. Consequently, DMRPNOCas enables in vivo dynamic monitoring of pulmonary macrophages, uncovering extensive recruitment and M1 polarization of monocyte-derived macrophages within 48 h of IAV infection. This process is accompanied by a significant reduction in alveolar macrophages. These findings provide new insights into macrophage-mediated pulmonary inflammation and underscore the potential of dual-locked probes for precise diagnosis and monitoring of pathological processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


