可见光诱导喹诺啉-2(1H)-酮序贯氮插入和苯并三唑化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报道了一个可见光介导的顺序反应,包括通过氮插入从苯1,2-二胺和亚硝基叔丁酯原位生成苯并三唑,并在一个锅中通过C-N键形成与喹诺啉-2(1H)- 1交叉偶联。该方案使用无金属温和条件,并展示了36个≤80%产率的例子,具有广泛的官能基耐受性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
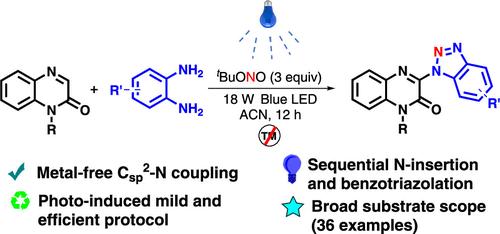
Visible Light-Induced Sequential Nitrogen Insertion and Benzotriazolation of Quinoxaline-2(1H)-ones
Herein, we report a visible light-mediated sequential reaction involving in situ generation of benzotriazole from benzene-1,2-diamine and tert-butyl nitrite via nitrogen insertion and concomitant cross-coupling with quinoxaline-2(1H)-ones via C–N bond formation in one pot. This protocol uses metal-free mild conditions and demonstrated 36 examples of ≤80% yield with wide functional group tolerance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


