Pd/手性磷酸激活不对称分子内双碳氢活化反应合成p -立体苯并膦孔氧化物
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种pd催化的分子内不对称双C-H活化反应,并确定了一种基于BINOL骨架的手性磷酸(CPA)是这种转化的合适配体。广泛的p -立体苯并膦孔氧化物以中等至优异的产率合成,具有高水平的对映选择性(≤96% ee)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
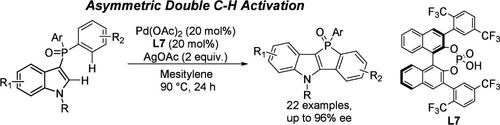
Pd/Chiral Phosphoric Acid-Enabled Asymmetric Intramolecular Double C–H Activation Reaction for the Synthesis of P-Stereogenic Benzophosphole Oxides
A Pd-catalyzed intramolecular asymmetric double C–H activation reaction was reported, and a BINOL skeleton-based chiral phosphoric acid (CPA) was identified as a suitable ligand for this transformation. A broad range of P-stereogenic benzophosphole oxides were synthesized in moderate to excellent yield with high levels of enantioselectivity (≤96% ee).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


