通过翻译后 O 到 C 的酰基转移编辑肽骨
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
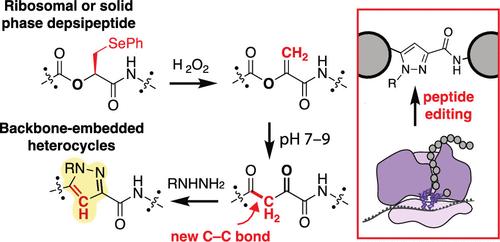
Peptide Backbone Editing via Post-Translational O to C Acyl Shift
Despite tremendous efforts to engineer translational machinery, replacing the encoded peptide backbone with new-to-nature structures remains a significant challenge. C, H, O, and N are the elements of life, yet ribosomes are capable of forming only C–N bonds as amides, C–O bonds as esters, and C–S bonds as thioesters. There is no current strategy to site-selectively form C–C bonds as ketones embedded in the backbones of ribosomal products. As an alternative to direct ribosomal C–C bond formation, here we report that peptides containing a dehydrolactic acid motif rapidly isomerize to generate backbone-embedded α,γ-diketoamides via a spontaneous formal O to C acyl shift rearrangement. The dehydrolactic acid motif can be introduced into peptides ribosomally or via solid-phase synthesis using α-hydroxyphenylselenocysteine followed by oxidation. Subsequent incubation at physiological pH produces an α,γ-diketoamide that can be diversified using a variety of nucleophiles, including hydrazines and hydroxylamines, to form pyrazoles and oximes, respectively. All of these groups remain embedded directly within the polypeptide backbone. This general strategy for peptide backbone editing, predicated on an intricate cascade of acyl rearrangements, provides the first nonenzymatic example of a C–C bond forming reaction to take place within a peptide backbone. The products so-produced are easily diversified into protein-like materials with backbone-embedded heterocycles. Application of this peptide editing strategy should accelerate the discovery of genetically encoded molecules whose properties more closely resemble those of bioactive natural products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


