由 N-杂环烯衍生的异构氮烯基碳心自由基
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
Azulene(与萘的同分异构体)是一种典型的非交替烃,由于其独特的电子结构和物理化学性质不同于传统的交替烃,因此作为π扩展分子的组成部分受到了广泛的关注。然而,利用azulene片段的稳定碳中心自由基的开发仍然相对较少。本文报道了氮杂环碳烯(NHCs)分别附着在氮杂环七元环的6位和五元环的2位上而设计合成的氮杂环基碳中心自由基异构体1和2的电子结构和光学性质。密度泛函理论计算表明,1中的自旋密度主要集中在azulene的五元环上,为π扩展环戊二烯基自由基;而2中的自旋密度主要分布在azulene的七元环上,为π扩展环庚三烯基自由基。此外,虽然自由基1和2都表现出反kasha发射,但与1 (λem ~ 396 nm)相比,2 (λem ~ 495 nm)的发射波长明显红移。本文章由计算机程序翻译,如有差异,请以英文原文为准。
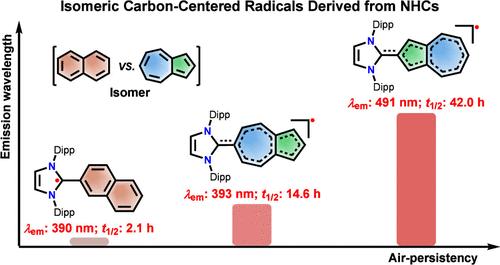
Isomeric Azulene-Based Carbon-Centered Radicals Derived from N-Heterocyclic Carbenes
Azulene (isomer with naphthalene), a representative nonalternant hydrocarbon, has attracted significant attention as a building block for π-extended molecules owing to its distinctive electronic structure and physicochemical properties that differ from those of conventional alternant hydrocarbons. Nevertheless, the development of stable carbon-centered radicals utilizing an azulene moiety remains relatively scarce. Herein, we report the electronic structures and optical properties of azulene-based carbon-centered radical isomers, 1 and 2, which were designed and synthesized by attaching N-heterocyclic carbenes (NHCs) to the 6-position of the seven-membered ring or 2-position of the five-membered ring of azulene, respectively. Density functional theory calculations reveal that the spin density in 1 is primarily localized on the five-membered ring of the azulene, indicating it as π-extended cyclopentadienyl radicals, whereas the spin density is predominantly distributed in the seven-membered ring of azulene for 2, serving as an example of π-extended cycloheptatrienyl radicals. Furthermore, although both radicals 1 and 2 exhibit anti-Kasha emission, the emission wavelength of 2 (λem ∼ 495 nm) is significantly red-shifted compared to that of 1 (λem ∼ 396 nm).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


