内生蒲公英球茎植物中的免疫抑制性 Secopenitrem 型吲哚二萜类化合物 Pochobulbins A-F
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
从内生真菌球孢(Pochonia bulbillosa KNI755中分离得到3个单clavatol- secopenittem(1-3)、1个双clavatol- secopenittem(4)和2个双-5-甲基邻苯二酚- secopenittem(5和6)。Pochobulbins a - f(1-6)是具有4/6/6/5/5/6/6/6/6/6融合十环体系的第二萜型吲哚二萜的初始实例。化合物1-4具有通过四氢- 2h -吡喃桥与clavatol单元融合的二烯基骨架。化合物5和6是第一个通过1,4-二氧六烷桥接5-甲基邻苯三酚单元和二苯三酚单元的二苯二萜型吲哚二萜。通过全面的光谱分析和ECD模拟,确定了完整的结构,包括它们的绝对立体化学分配。化合物1、3、5对T细胞增殖有抑制作用,化合物1、3对B细胞增殖也有抑制作用,EC50值在4.6 ~ 13 μM之间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
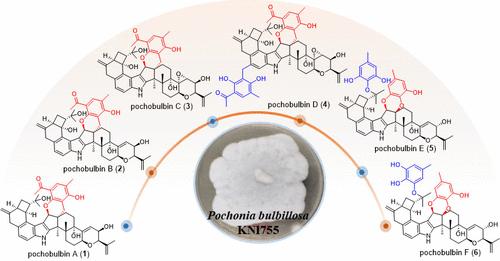
Pochobulbins A–F, Immunosuppressive Secopenitrem-Type Indole Diterpenoids from an Endophytic Pochonia bulbillosa
Three monoclavatol-secopenitrems (1–3), one bis-clavatol-secopenitrem (4), and two bis-5-methylpyrogallol-secopenitrems (5 and 6) were obtained from the endophytic fungus Pochonia bulbillosa KNI755. Pochobulbins A–F (1–6) are the initial instances of secopenitrem-type indole diterpenoids with a 4/6/6/5/5/6/6/6/6/6 fused decacyclic ring system. Compounds 1–4 feature a secopenitrem skeleton fused to a clavatol unit via a tetrahydro-2H-pyran bridge. Compounds 5 and 6 are the first secopenitrem-type indole diterpenoids that connect a 5-methylpyrogallol unit to a secopenitrem unit via a 1,4-dioxane bridge. The complete structures, including their absolute stereochemical assignments, were determined through comprehensive spectroscopic analyses and ECD simulations. Compounds 1, 3, and 5 inhibited T cell proliferation, and compounds 1 and 3 also inhibited B cell proliferation, exhibiting EC50 values between 4.6 and 13 μM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


