在产肠毒素大肠杆菌疫苗研究中,使用TaqMan阵列卡直接从粪便样本中鉴定肠毒素和定植因子。
摘要
产肠毒素大肠杆菌(ETEC)是儿童和旅行者腹泻的主要原因。候选疫苗ETVAX包含几种ETEC定植因子(CFs),其中混合LT(热不稳定毒素)/霍乱毒素B亚基与双突变LT佐剂。来自2b期ETVAX试验的粪便样本通过基于pcr的定制TaqMan阵列卡(TAC)进行测试,包括三个ETEC毒素基因(LT和热不稳定毒素,STh和STp)和18个ETEC CFs。对粪便样品进行扩增和培养分子平台检测,然后进行gm1酶联免疫吸附试验(ELISA)和抑制gm1酶联免疫吸附试验(ELISA),并对6株(最多)培养株中鉴定的etec进行CFs斑点印迹检测。与Amplidiag相比,TAC检测ETEC的灵敏度为89.4%(320/358),特异性为96.4%(405/420)。两种方法具有良好的定量相关性(定量周期R2 = 0.827, P < 0.05)。与培养相比,TAC和Amplidiag的灵敏度分别为96.8%(184/190),在588个培养阴性的粪便中分别鉴定出151个和174个PCR阳性。大肠杆菌大肠杆菌(ETEC)菌落LT和STh/STp与ELISA的一致性为85.5%(165/193)。与793个菌落的ETVAX CFs CFA/I、CS3、CS5和CS6的点印迹结果相比,TAC的灵敏度为98%,特异性为92%。总体上,335例(43.1%)标本通过TAC检测到ETEC, 358例(46.0%)标本通过扩增检测到ETEC,而培养190例(24.4%)标本通过扩增检测到ETEC。我们的结论是,与培养相比,TAC或Amplidiag的分子诊断方法增加了ETEC的检测,TAC也可以提供疫苗亚型ETEC的数据。临床试验:该研究已在ClinicalTrials.gov注册为NCT03729219。重要意义产肠毒素大肠杆菌(ETEC)是儿童和旅行者腹泻的重要原因。正在开发的疫苗利用特定毒素和定植因子(CFs)作为抗原。因此,无论是用于疫苗试验还是指导流行病学,都需要临床微生物学诊断方法来区分特定毒素和CFs。在这项工作中,我们评估了几种方法对ETEC的诊断性能:基于pcr的定制TaqMan阵列卡(TAC)和粪便和大肠杆菌培养的分子平台Amplidiag,然后是gm1酶联免疫吸附法检测毒素和斑点斑点法检测CFs。使用2b期ETEC疫苗试验的粪便样本。总体而言,335个样本(43.1%)通过TAC检测到ETEC, 358个样本(46.0%)通过扩增检测到ETEC,而培养190个样本(24.4%)检测到ETEC。TAC还提供了CF数据,灵敏度为98%,特异性为92%。我们的结论是,与培养相比,TAC或ampliag的分子诊断方法增加了ETEC的检测。
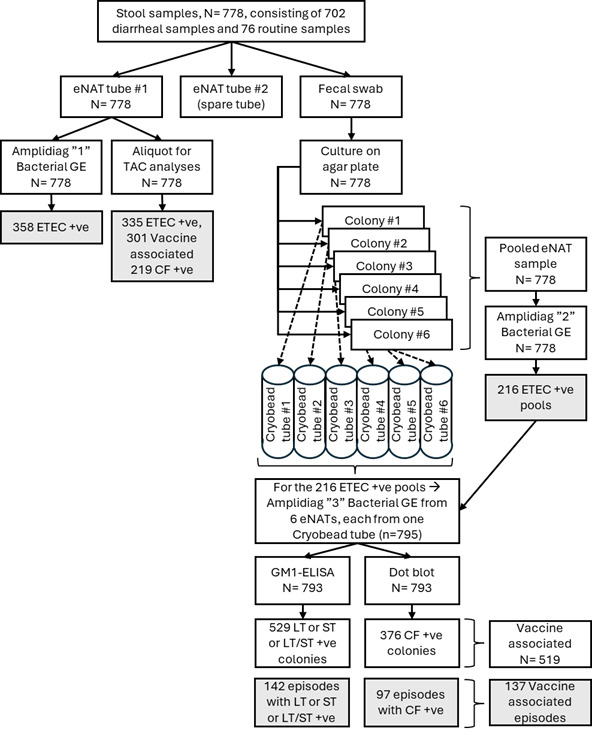
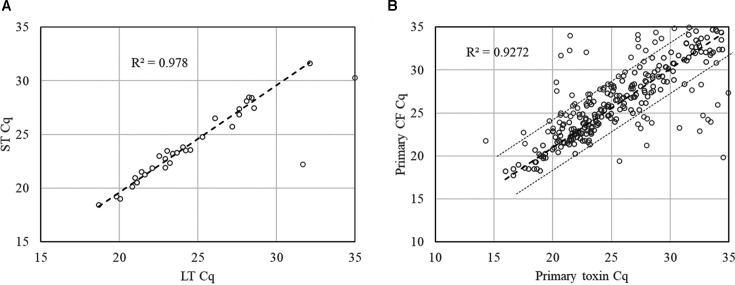
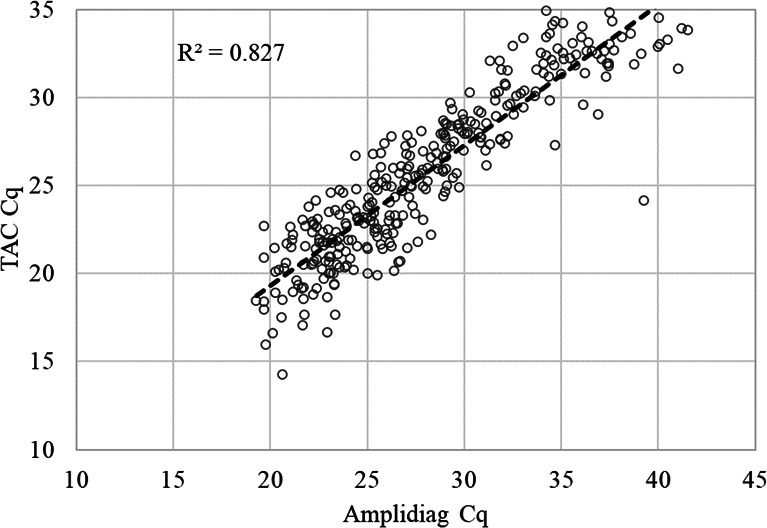
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of childhood and travelers' diarrhea. The vaccine candidate ETVAX encompasses several ETEC colonization factors (CFs) with a hybrid LT (heat-labile toxin)/cholera toxin B subunit adjuvanted with a double-mutant LT. Stool samples from a Phase 2b ETVAX trial were tested by a PCR-based customized TaqMan Array Card (TAC), including three ETEC toxin genes (LT and heat-stable toxins, STh and STp) and 18 ETEC CFs. Stool samples were also tested with the molecular platform Amplidiag and culture, followed by GM1-enzyme-linked immunosorbent assay (ELISA) and inhibition GM1-ELISA for LT and ST and dot blot for CFs of ETECs identified among six culture isolates (maximum). Compared with Amplidiag, TAC yielded 89.4% sensitivity (320/358) and 96.4% specificity (405/420) for ETEC detection. The two methods demonstrated a good quantitative correlation (quantification cycle R2 = 0.827, P < 0.05). Compared with culture, TAC and Amplidiag each exhibited 96.8% (184/190) sensitivity and identified an additional of 151 and 174 PCR positives in 588 culture-negative stools, respectively. The concordance of stool TAC versus ELISA of ETEC colonies for LT and STh/STp was 85.5% (165/193). TAC demonstrated 98% sensitivity and 92% specificity versus the dot blot results of 793 colonies for the ETVAX CFs CFA/I, CS3, CS5, and CS6. Overall ETEC was detected by TAC in 335 (43.1%) and by Amplidiag in 358 (46.0%) of specimens compared to 190 (24.4%) by culture. We conclude that molecular diagnostic approaches of TAC or Amplidiag increase the detection of ETEC compared with culture, and TAC can also provide vaccine-subtype ETEC data.CLINICAL TRIALSThis study was registered with ClinicalTrials.gov as NCT03729219.IMPORTANCEEnterotoxigenic Escherichia coli (ETEC) is an important cause of childhood and travelers' diarrhea. Vaccines in development utilize specific toxins and colonization factors (CFs) as antigens. Therefore, clinical microbiologic diagnostic methods are needed to discriminate specific toxins and CFs, both for vaccine trials and to guide epidemiology. In this work, we assessed the diagnostic performance of several methods for ETEC: a PCR-based customized TaqMan Array Card (TAC) and the molecular platform Amplidiag on stool and E. coli culture, followed by GM1-enzyme-linked immunosorbent assay for toxins and dot blot for CFs. Stool samples from a Phase 2b ETEC vaccine trial were used. Overall, ETEC was detected by TAC in 335 (43.1%) and by Amplidiag in 358 samples (46.0%) compared to 190 (24.4%) by culture. TAC additionally provided CF data with 98% sensitivity and 92% specificity. We conclude that the molecular diagnostic approaches of TAC or Amplidiag increase the detection of ETEC compared with culture.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


