trna的自由内含子作为互补依赖的基因表达调节因子
IF 16.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从古细菌到人类,转移RNA (tRNA)基因的一个子集拥有一个内含子,必须从转录的前tRNA中去除该内含子才能产生成熟的、功能性的tRNA。tRNA内含子序列的进化守恒表明,tRNA内含子具有序列依赖的细胞功能,目前尚不清楚。在这里,我们证明了酿酒酵母中trna的自由内含子(fitRNAs)作为小的调控rna,通过与mRNA编码区(接近)完美互补的长(13 - 15nt)统计上不可能的延伸来抑制mRNA水平。由于tRNAIle内含子的基因组缺失或诱导过表达会导致相应的互补mrna水平的增加或减少,因此fitrna的功能既是组成性的,也是可诱导的。值得注意的是,尽管tRNA内含子通常被迅速降解,但氧化应激后,fitRNATrp选择性地积累,靶mRNA水平下降。因此,fitRNAs作为基因调节剂,可以微调基础mRNA表达并改变响应氧化应激的mRNA网络。本文章由计算机程序翻译,如有差异,请以英文原文为准。
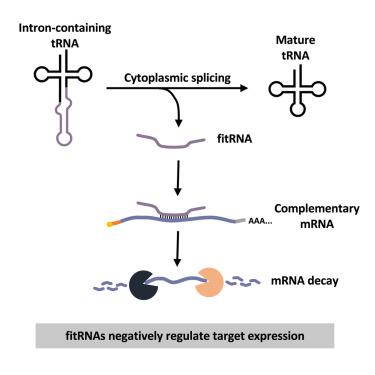
Free introns of tRNAs as complementarity-dependent regulators of gene expression
From archaea to humans, a subset of transfer RNA (tRNA) genes possesses an intron that must be removed from transcribed pre-tRNAs to generate mature, functional tRNAs. Evolutionary conservation of tRNA intron sequences suggests that tRNA introns perform sequence-dependent cellular functions, which are presently unknown. Here, we demonstrate that free introns of tRNAs (fitRNAs) in Saccharomyces cerevisiae serve as small regulatory RNAs that inhibit mRNA levels via long (13–15 nt) statistically improbable stretches of (near) perfect complementarity to mRNA coding regions. The functions of fitRNAs are both constitutive and inducible because genomic deletion or inducible overexpression of tRNAIle introns led to corresponding increases or decreases in levels of complementary mRNAs. Remarkably, although tRNA introns are usually rapidly degraded, fitRNATrp selectively accumulates following oxidative stress, and target mRNA levels decrease. Thus, fitRNAs serve as gene regulators that fine-tune basal mRNA expression and alter the network of mRNAs that respond to oxidative stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


