β-氯丙烯醛与间氨基噻吩的生物正交反应制备同时双色成像近红外荧光探针
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
基于高荧光探针的生物正交化学已成为一种很有前途的生物医学应用工具。然而,大多数荧光探针都是通过在经典荧光团中引入生物正交伴侣作为荧光猝灭剂来设计的,随着发射波长向近红外(NIR)区域移动,这些探针的荧光性会逐渐恶化,这极大地限制了它们在体内的应用。在此,我们报道了一种新的荧光生物正交反应,涉及β-氯丙烯醛(β-CAs)和间氨基噻吩(m-AT1),其荧光在原位产生荧光团后增加了500倍以上。β-CAs在生理条件下稳定,在生物亲核试剂存在下与m-AT1发生快速反应(β-CA9, k2 = 2.2 × 102 M-1 s-1,在H2O中)和化学选择性反应,即使在无溶剂条件下,反应也进行得很快。此外,通过调节β-CAs的共轭长度,可以使探针的发射波长在627 ~ 778 nm之间进行微调。这些探针允许同时标记多个细胞器而无需洗涤步骤,并且在活体小鼠中实现双色肿瘤可视化。我们相信,该研究不仅为开发具有优越开启行为的近红外荧光探针提供了新的见解,而且还为生物医学研究提供了一种具有广泛应用潜力的荧光生物正交反应CA-AT。本文章由计算机程序翻译,如有差异,请以英文原文为准。
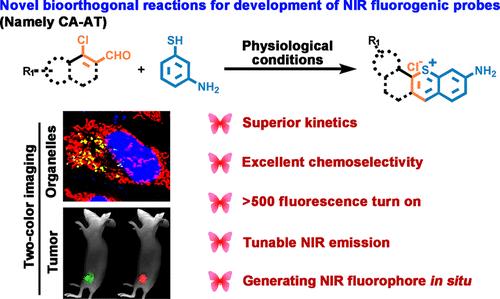
Bioorthogonal Reaction of β-Chloroacroleins with meta-Aminothiophenol to Develop Near-Infrared Fluorogenic Probes for Simultaneous Two-color Imaging
Highly fluorogenic probe based bioorthogonal chemistry has become a promising tool in biomedical applications. However, the majority of fluorogenic probes are designed by introducing a bioorthogonal partner as a fluorescence quencher into classical fluorophores, and these probes exhibit a deteriorating fluorogenicity as the emission wavelength shifts toward the near-infrared (NIR) region, greatly limiting their applications in vivo. Herein, we report a novel fluorogenic bioorthogonal reaction involving β-chloroacroleins (β-CAs) and meta-aminothiophenol (m-AT1), whose fluorescence increases more than 500-fold upon in situ generating fluorophores. β-CAs are stable under physiological conditions and react rapidly (β-CA9, k2 = 2.2 × 102 M–1 s–1, in H2O) and chemoselectively with m-AT1 in the presence of biological nucleophiles, and delightfully, the reaction proceeds swiftly even under solvent-free conditions. Furthermore, manipulating the conjugate length of β-CAs enables the emission wavelength of the probes to be fine-tuned from 627 to 778 nm. These probes allow the simultaneous labeling of multiple cellular organelles without washing steps, and two-color tumor visualization is achieved in living mice. We believe this study not only provides new insights for the development of NIR fluorogenic probes with superior turn-on behaviors but also presents a promising fluorogenic bioorthogonal reaction CA-AT with widespread potential applications in biomedical research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


