In(OTf)3- only通过活化附酰胺辅助基团的炔催化糖基化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们在此提出了一种新的糖基化方案,使用糖基o-N-(o-甲氧基苄基)-丙基苯甲酸酯作为供体,仅由催化量的In(OTf)3激活,不需要Au(I)催化剂。这种方法提供了温和和正交的条件,使糖基化能够跨越广泛的底物范围,并促进了聚糖组装的通用一锅策略。机理分析表明,In(OTf)3具有双重作用,通过σ-配位被吸收到辅助基团中,同时通过π-相互作用激活炔。本文章由计算机程序翻译,如有差异,请以英文原文为准。
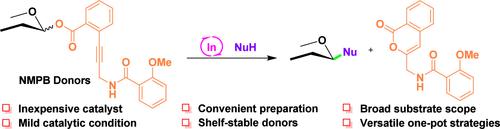
In(OTf)3-Only-Catalyzed Glycosylation via Activation of an Alkyne Appended with an Amide Auxiliary Group
We herein present the development of a novel glycosylation protocol using glycosyl o-N-(o-methoxybenzamidoyl)-propynyl benzoates as donors, activated solely by a catalytic amount of In(OTf)3, eliminating the need for a Au(I) catalyst. This approach offers mild and orthogonal conditions, enabling glycosylation across a broad substrate scope and facilitating versatile one-pot strategies for glycan assembly. Mechanistic insights suggest that In(OTf)3 serves dual roles by being recruited to the auxiliary group through σ-coordination while activating the alkyne via π-interaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


