为什么弱结合M-N-C单原子催化剂具有异常高的氧还原活性?
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
具有金属-氮-碳(M-N-C)结构的单原子催化剂(SACs)在氧还原反应(ORR)中具有广泛的应用前景。根据经典的Sabatier原理,最佳的三维金属催化剂,如Fe/ Co-N-C,由于结合强度适中,具有优越的催化性能。然而,弱结合M-N-C催化剂(如Ni/ Cu-N-C)所显示的大量ORR活性挑战了目前的认识,强调了探索新的潜在机制的必要性。在这项工作中,我们将ph场耦合微动力学模型与详细的实验电子态分析相结合,以验证弱结合SACs ORR反应途径中的一个新的关键步骤──金属-氮桥位点的氧吸附。这一步骤显著改变了吸附结垢关系、电场响应和溶剂化效应,进一步影响了从HOO*到O*的关键动力学反应屏障和ph依赖性性能。同步加速器光谱分析进一步为新的弱结合M-N-C模型提供了证据,表明在弱结合M-N-C催化剂中,N原子的反键π轨道上的电子密度增加,并证实了N - o键的存在。这些发现重新定义了对弱结合M-N-C催化剂行为的理解,为其在清洁能源中的应用开辟了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
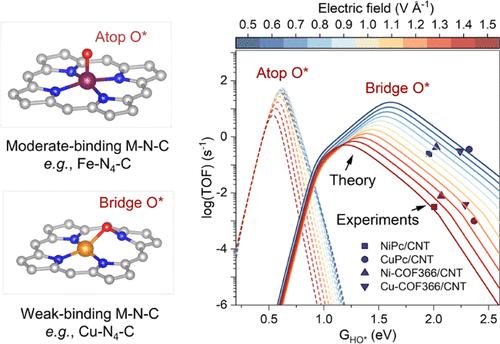
Why Do Weak-Binding M–N–C Single-Atom Catalysts Possess Anomalously High Oxygen Reduction Activity?
Single-atom catalysts (SACs) with metal–nitrogen–carbon (M–N–C) structures are widely recognized as promising candidates in oxygen reduction reactions (ORR). According to the classical Sabatier principle, optimal 3d metal catalysts, such as Fe/Co–N–C, achieve superior catalytic performance due to the moderate binding strength. However, the substantial ORR activity demonstrated by weakly binding M–N–C catalysts such as Ni/Cu–N–C challenges current understandings, emphasizing the need to explore new underlying mechanisms. In this work, we integrated a pH-field coupled microkinetic model with detailed experimental electron state analyses to verify a novel key step in the ORR reaction pathway of weak-binding SACs─the oxygen adsorption at the metal–nitrogen bridge site. This step significantly altered the adsorption scaling relations, electric field responses, and solvation effects, further impacting the key kinetic reaction barrier from HOO* to O* and pH-dependent performance. Synchrotron spectra analysis further provides evidence for the new weak-binding M–N–C model, showing an increase in electron density on the antibonding π orbitals of N atoms in weak-binding M–N–C catalysts and confirming the presence of N–O bonds. These findings redefine the understanding of weak-binding M–N–C catalyst behavior, opening up new perspectives for their application in clean energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


