立体选择性化学酶级联反应合成密集功能化亚氨基糖
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
1,4-二羰基是构建多种药物载体和天然产物的多功能合成物。然而,立体选择性合成密集功能化1,4-二羰基是具有挑战性的。在这里,我们报道了一种多用途的生物催化途径,利用d-果糖-6-磷酸醛缩酶(EcFSA)以高产量和高达克级的速度获得手性2,3-二羟基-1,4-二酮。这些化合物作为合子的效用在酶级联反应中得到例证,随后的区域和立体选择性酶转氨化反应形成密集功能化的同手性1-吡咯啉,然后化学或酶还原为四取代吡咯烷。本文章由计算机程序翻译,如有差异,请以英文原文为准。
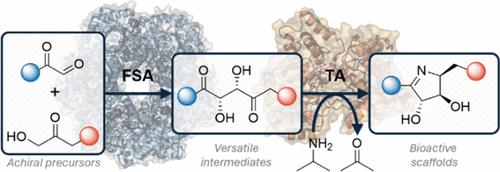
Stereoselective Chemoenzymatic Cascades for the Synthesis of Densely Functionalized Iminosugars
1,4-Dicarbonyls are versatile synthons for the construction of diverse pharmacophores and natural products. However, the stereoselective synthesis of densely functionalized 1,4-dicarbonyls is challenging. Here, we report a versatile biocatalytic route to access chiral 2,3-dihydroxy-1,4-diketones in high yields and up to gram scale using d-fructose-6-phosphate aldolase (EcFSA). The utility of these compounds as synthons is exemplified in enzyme cascades with subsequent regio- and stereoselective enzymatic transamination to form densely functionalized homochiral 1-pyrrolines followed by chemical or enzymatic reduction to tetrasubstituted pyrrolidines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


