丙烯醛介导的赖氨酸转化为亲电杂环的蛋白质多样化和毒性分析
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
了解存在生物代谢物的蛋白质相互作用对于揭示生物过程和推进治疗干预至关重要。本研究的重点是α,β-不饱和羰基,特别是丙烯醛衍生的蛋白质修饰,揭示了一锅,四步,选择性化学反应,导致形成杂环α,β-不饱和羰基,称为3-甲酰基-3,4-脱氢piperidino (FDP),仅在赖氨酸残基上。值得注意的是,这种化学反应将亲核试剂赖氨酸转化为亲电战斗部。我们证明了它在后期多肽多样化,精确蛋白质工程和均匀蛋白质标记与不同有效载荷的多功能性。此外,fdp -赖氨酸在无试剂条件下通过脱氧和芳构化顺利转化为另一种杂环3-甲基吡啶(3-MP)赖氨酸。这种转化促进了后期肽功能化和蛋白的均质工程,mp -赖氨酸作为质量助推器。利用这种化学反应,我们通过FDP和mp修饰蛋白的化学蛋白质组学分析发现了负责丙烯醛诱导修饰的高反应位点。我们的研究结果揭示了fdp修饰蛋白介导的蛋白-蛋白相互作用的变化,并发现了fdp修饰蛋白的约1548个新的交联伙伴。本文章由计算机程序翻译,如有差异,请以英文原文为准。
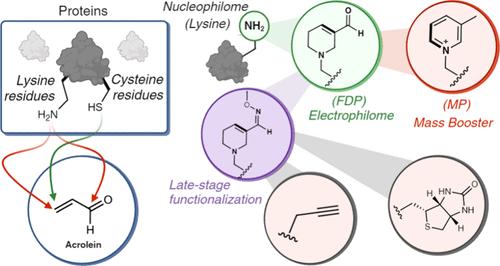
Acrolein-Mediated Conversion of Lysine to Electrophilic Heterocycles for Protein Diversification and Toxicity Profiling
Understanding protein interactions in the presence of biological metabolites is critical for unraveling biological processes and advancing therapeutic interventions. This study focuses on α,β-unsaturated carbonyls, particularly acrolein-derived protein modifications, unveiling a one-pot, four-step, selective chemistry that results in the formation of a heterocyclic α,β-unsaturated carbonyl, termed 3-formyl-3,4-dehydropiperidino (FDP), exclusively on lysine residues. Remarkably, this chemistry transforms lysine, a nucleophile, into an electrophilic warhead. We demonstrate its versatility in late-stage peptide diversification, precision protein engineering, and homogeneous protein labeling with diverse payloads. Additionally, FDP-lysine smoothly transforms into another heterocycle, 3-methylpyridinium (3-MP) lysine via deoxygenation and aromatization in reagentless conditions. This transformation facilitates late-stage peptide functionalization and homogeneous engineering of proteins, with MP-lysine acting as a mass booster. Leveraging this chemistry, we discovered hyperreactive sites responsible for acrolein-induced modification through chemoproteomic profiling of FDP- and MP-modified proteins. Our findings revealed changes in protein–protein interactions mediated by FDP-modified proteins and uncovered ∼1548 novel cross-linking partners of an FDP-modified protein.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


