咔唑的合成:硝基芳烃与格氏试剂的光催化串联偶联
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d5qo00019j
引用次数: 0
摘要
本研究报道了一种以硝基芳烃和格氏试剂为原料,通过分子间热C-N键偶联,光诱导氮杂氮-6π电环化反应,一锅合成咔唑的有效方法。光化学途径只需要用紫光(390 ~ 395 nm)照射反应混合物,不需要任何外部催化剂和添加剂,从而为合成咔唑提供了一条新颖的、阶梯经济的途径。机理研究表明,亚硝基芳烃和二芳胺是反应过程中重要的中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
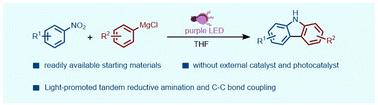
Synthesis of carbazoles: light-promoted tandem coupling of nitroarenes with Grignard reagents†
In this study, an efficient method for one-pot synthesis of carbazoles from readily available nitroarenes and Grignard reagents by intermolecular thermal C–N bond coupling followed by a photoinduced aza-6π electrocyclization reaction is reported. The photochemical pathway only requires irradiation of the reaction mixture with purple light (390–395 nm), without any external catalysts and additives, thus providing a novel and step-economic route for the synthesis of carbazoles. Mechanistic studies suggest that nitrosoarene and diarylamine are important intermediates in the reaction process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


