半夹层稀土催化剂催化内炔与端炔的区域选择性和立体选择性加氢烷基化反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
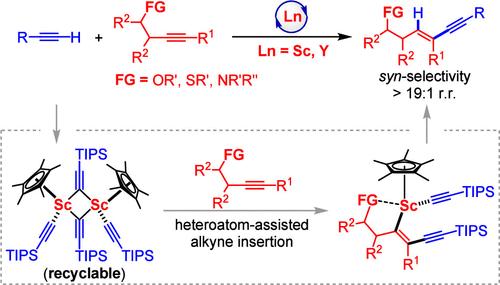
内炔与末端炔的区域选择性和立体选择性氢炔基化反应是合成多取代1,3-炔的一种直接途径,具有重要意义。然而,当使用不对称的内炔时,这种转化通常会遇到区域选择性和立体选择性问题。本文首次报道了在半夹心稀土催化剂作用下,异丙基醚、硫醚和叔胺等多种杂原子功能化的不对称内炔与末端炔的区域和同步立体选择性氢烷基化反应。该方案为合成新的杂原子(O, S或N)功能化1,3-炔提供了一个原子效率和直接的途径,具有100%的原子效率,广泛的底物范围,高区域和同步立体选择性(>;19:1 rr和>;19:1 syn/anti)。机理细节已通过氘标记实验、对照实验、关键反应中间体的分离和转化得到阐明。揭示了该反应是通过半夹心式烷基钪对末端炔的C(sp) -H去质子化,形成具有催化活性的二聚体半夹心式烷基钪,然后在杂原子的辅助下将内部炔插入到sc -炔基键中,然后将生成的sc -炔基键与另一个末端炔分子进行质子解。内部炔的杂原子(O, S或N)与催化剂金属中心的配位在实现高水平的反应活性和区域选择性和立体选择性方面起着至关重要的作用。值得注意的是,催化活性二聚体半夹层四炔基钪可以回收再利用,构成了内炔氢烷基化可回收催化剂体系的第一个例子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Regio- and Stereoselective Hydroalkynylation of Internal Alkynes with Terminal Alkynes by Half-Sandwich Rare-Earth Catalysts
The regio- and stereoselective hydroalkynylation of internal alkynes with terminal alkynes is of great interest and importance as a straightforward route for synthesizing multisubstituted 1,3-enynes. However, this transformation often suffers from regio- and stereoselectivity issues when working with unsymmetrical internal alkynes. Herein, we report for the first time the regio- and syn-stereoselective hydroalkynylation of a variety of heteroatom-functionalized unsymmetrical internal alkynes including homopropargyl ethers, thioethers, and tertiary amines with terminal alkynes by half-sandwich rare-earth catalysts. This protocol provides an atom-efficient and straightforward route for the synthesis of a new family of heteroatom (O, S, or N)-functionalized 1,3-enynes, featuring 100% atom-efficiency, broad substrate scope, and high regio- and syn-stereoselectivity (>19:1 r.r. and >19:1 syn/anti). The mechanistic details have been elucidated by deuterium-labeling experiments, control experiments, and isolation and transformations of key reaction intermediates, revealing that the reaction proceeded through the C(sp)–H deprotonation of a terminal alkyne by a half-sandwich scandium alkyl species to form a catalytically active dimeric half-sandwich scandium tetraalkynyl species followed by heteroatom-assisted insertion of internal alkyne into the Sc–alkynyl bond and the subsequent protonolysis of the resulting Sc–alkenyl bond with another terminal alkyne molecule. The coordination of the heteroatom (O, S, or N) of internal alkynes to the catalyst metal center plays a critically important role in achieving a high level of reactivity and regio- and stereoselectivity. Remarkably, the catalytically active dimeric half-sandwich scandium tetraalkynyl species can be recovered and reused, constituting the first example of a recyclable catalyst system for the hydroalkynylation of internal alkynes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


