纳米约束下可逆环加成的单电子催化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
电子转移(ET)在许多化学反应中起着至关重要的作用,但由于反应种类和途径的复杂性,其机理和作用在纳米生物技术中尚不清楚。通过在单分子水平上调制和监测电子行为,我们可以更好地理解控制它们的基本机制和方法。在这里,我们揭示了在带正电的纳米约束下单电子催化的机制。我们证明了(2 + 2)和(4 + 4)环加成都可以被一个电子可逆地催化。通过先进的单分子检测平台监测环加成过程中的顺序电信号,发现关键的反应途径。实验和理论结果一致表明,将单一ET过程与含有瓜b[8]uril的纳米约束相结合,可以降低反应能垒,促进可逆环加成。此外,我们还证明了偏置电压可以微调ET过程和键形成和解理的化学平衡。我们的研究结果为解释、调节和设计电子反应和功能化器件提供了一种新的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
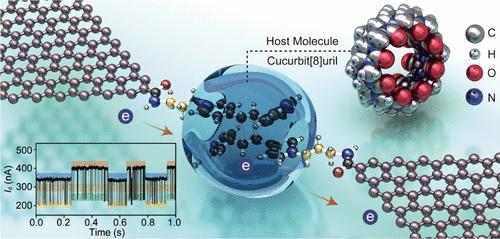
Single-Electron Catalysis of Reversible Cycloadditions under Nanoconfinement
Electron transfer (ET) is crucial in many chemical reactions, but its mechanism and role are hardly understood in nanobiotechnology due to the complexity of reaction species and pathways involved. By modulating and monitoring electron behavior at the single-molecule level, we can better understand the fundamental mechanisms and ways to control them for technological use. Here, we unravel a mechanism of single-electron catalysis under positively charged nanoconfinement. We demonstrate that both (2 + 2) and (4 + 4) cycloadditions can be catalyzed reversibly by a single electron. Key reaction pathways are discovered by monitoring sequential electrical signals in the cycloadditions through advanced single-molecule detection platforms. Experimental and theoretical results consistently demonstrate that combining single ET processes with nanoconfinement involving cucurbit[8]uril can lower the reaction energy barrier and promote reversible cycloaddition. Moreover, we show that the bias voltage can fine-tune ET processes and chemical equilibria in bond formation and cleavage. Our results provide a novel approach to elucidate, modulate, and design electron-involved reactions and functionalized devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


