有机半导体的三维电子衍射结构揭示了构象多态性
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
晶体和构象多态性在材料的物理和化学性质中起着至关重要的作用,影响它们的稳定性、溶解度和生物利用度,这对于制药、材料科学和化学的各种应用至关重要。尽管具有重要意义,但这些多态性的结构分析,特别是构象多态性,仍然具有挑战性,因为有限的方法可以为多态性的微晶变体提供足够的分辨率。三维电子衍射(3D ED)是一种新兴的技术,在阐明功能有机分子、药物和生物分子的微晶体结构方面具有重要的潜力。尽管有这种潜力,但小分子的3D ED结构的有限实例显示出最低的晶体对称性,具有首选取向和可能的组成分子构象变化。一种新型有机半导体,ph -抗苯并噻吩[5,6-b]苯并噻吩[3,2-b]噻吩- c10 (antiC10),就是这样的例子之一。我们成功地确定了这个具有挑战性的分子的3D ED结构。antiC10晶体表现出最低的对称性(空间群P1),并且相对于网格的首选方向导致锥体缺失。通过采用从头生成搜索模型的顺序分子替换方法克服了这些挑战。所得的八聚体anti10结构揭示了两层单层结构和反平行的烷基间指人字形结构,而不是之前报道的全平行的同分异构体。同时,烷基链相互交错,位于相邻的π核地层之间。详细分析了两层分子间的人字形排列和分子内构象的多态性。具有构象多态性的结构可能处于亚稳态中间状态,通过孪晶稳定。这些发现可能为有机半导体的结晶机制和合理设计提供重要的见解。这项研究表明,3D ED技术和顺序相位方法的进步使得研究以前无法到达的结构成为可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
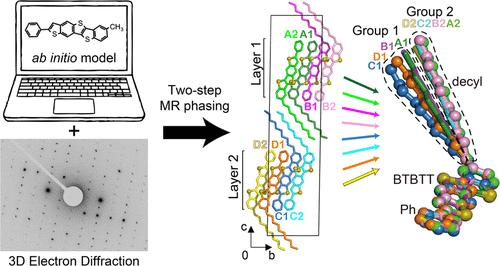
3D Electron Diffraction Structure of an Organic Semiconductor Reveals Conformational Polymorphism
Crystal and conformational polymorphisms play crucial roles in the physical and chemical properties of materials, impacting their stability, solubility, and bioavailability, which are essential for various applications in pharmaceuticals, materials science, and chemistry. Despite their significance, the structural analysis of these polymorphisms, particularly conformational polymorphisms, remains challenging due to the limited methodology that provides sufficient resolution for microcrystalline variants of polymorphs. Three-dimensional electron diffraction (3D ED) is an emerging technique with significant potential for elucidating the microcrystal structures of functional organic molecules, pharmaceuticals, and biomolecules. Despite this potential, there are limited instances of 3D ED structures for small molecules exhibiting the lowest crystallographic symmetry with a preferred orientation and possibly conformational variations of constituent molecules. A novel organic semiconductor, Ph-anti-benzothieno[5,6-b]benzothieno[3,2-b]thiophene-C10 (antiC10), is one of such examples. We successfully determined the 3D ED structure of this challenging molecule. The antiC10 crystal exhibited the lowest symmetry (space group P1), and the preferred orientations against the grid resulted in a missing cone. These challenges were surmounted by employing a sequential molecular replacement approach with an ab initio-generated search model. The resulting octameric antiC10 structure reveals a two-monolayer architecture and an antiparallel alkyl-interdigitated herringbone configuration in contrast to the all-parallel associations observed in its previously reported isomer. Concurrently, the alkyl chains are intricately interdigitated with each other and positioned between the adjacent π-core strata. Detailed analysis has elucidated the conformational polymorphism in herringbone packing between the two monolayers as well as in intramolecular conformations among monomers. The structure with conformational polymorphism is presumably in a metastable intermediate state, stabilized by twinning. These findings may provide critical insights into the crystallization mechanisms and rational design of organic semiconductors. This research demonstrates that advancements in 3D ED technology and sequential phasing methodologies have enabled the study of previously unreachable structures.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


