通过生物启发离子通道设计加速非浓缩水溶液中的离子脱溶
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在基于水的电化学储能装置中,电化学界面水不受控制的水解限制了这种水性电池或超级电容器在商业上的应用。“盐包水”设计是拓宽水溶液电化学稳定性窗口的有效策略,但制造成本高、电解质粘度高等缺点也阻碍了其发展。在这里,受细胞膜生物离子通道的启发,我们提出了一种有效的方法来设计电极表面,在电化学界面诱导水合离子的脱溶,并抑制非浓缩电解质中的水分解。生物工程策略能够诱导可控的脱溶,并加速水合离子(如钾)的运输。亚纳米设计(0.8 nm)使得水合钾离子在水合数仅为0.3的情况下脱离其溶剂化壳,而孔基团与钾离子之间的静电相互作用促进了它们的运输。锌||电池在1ma cm-2 / 10mah cm-2下的稳定循环寿命超过1000小时。这项工作为低浓度水溶液中电化学界面的调节提供了新的思路,为设计基于水的储能装置提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
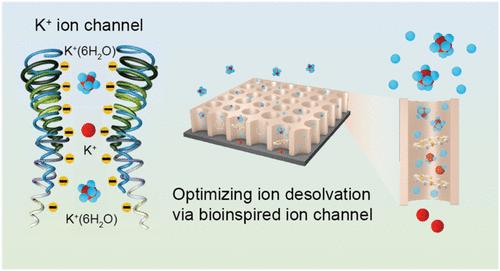
Accelerating Ion Desolvation via Bioinspired Ion Channel Design in Nonconcentrated Aqueous Electrolytes
In aqueous-based electrochemical energy storage devices, uncontrolled hydrolysis of water at the electrochemical interfaces limits the application of such aqueous batteries or supercapacitors in business. The “water-in-salt” design is a valid strategy to broaden the electrochemical stability window in aqueous electrolytes, but drawbacks such as high manufacturing cost, high electrolyte viscosity, etc., also hinder its development. Here, inspired by biological ion channels in cell membranes, we propose an effective approach to engineer the electrode surface, inducing the desolvation of hydrated ions at the electrochemical interface and inhibiting water decomposition in nonconcentrated electrolytes. The biological engineering strategy enables the induction of controlled desolvation and accelerates the transportation of hydrated ions, e.g., potassium. The subnanometer design (0.8 nm) forces the hydrated potassium ions to shed their solvation shell with a hydration number of only 0.3, while the electrostatic interactions between the pore groups and the potassium ions facilitate their transport. The Zn||Zn cells demonstrate a stable cycling lifespan of over 1000 h at 1 mA cm–2/10 mAh cm–2. This work sheds new light on regulating the electrochemical interfaces in low-concentration aqueous electrolytes for designing aqueous-based energy storage devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


