以CO2为开关试剂的2-芳基苯胺底物控制的发散还原环化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
CO2捕集在有机合成领域受到高度重视,特别是在双CO2转化方面。在这项研究中,我们详细介绍了在PMHS[聚甲基氢硅氧烷]存在下,用双CO2作为双官能试剂对2-吲哚苯胺进行的一种新的还原环化反应,产生甲基取代的喹诺啉。此外,2-吡咯苯胺的另一种化学选择性环化也通过转化一氧化碳实现。机制研究揭示了这样一个事实,即这种底物控制的分化主要取决于n -二乙酰化中间体的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
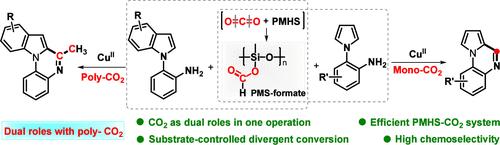
Substrate-Controlled Divergent Reductive Cyclization of 2-Arylanilines Using CO2 as a Switching Reagent
Capturing CO2 is highly valued in the field of organic synthesis, especially underdeveloped dual-CO2 conversion. In this study, we detail a novel reductive cyclization of 2-indolylanilines with dual CO2 as a difunctional reagent in the presence of PMHS [poly(methylhydrosiloxane)], delivering methyl-substituted quinoxalines. Furthermore, another chemoselective cyclization with 2-pyrrolylanilines is also realized by converting mono-CO2. Mechanistic investigations shed light upon the fact that this substrate-controlled divergence mainly depends on the formation of N-diacylative intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


