dmoa衍生美罗萜类化合物的全合成:选择性合成(+)- berkeley缩醛D和(+)- penici缩醛I
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
复杂天然产物的合成需要对化学选择性、立体选择性和区域选择性进行有效的控制。伯克利缩醛是3,5-二甲基苯甲酸(DMOA)衍生的巯基萜类化合物的一个亚家族,由于其密集功能化和高度氧化的结构,给合成带来了很大的挑战,这限制了合成的努力。在这里,我们首次合成了这类dmoa衍生的巯基萜类化合物,特别是(+)- berkeley缩醛D和(+)- penici缩醛i。我们的方法是化学选择性去质子化,然后是分子内单电子转移(SET),从烯酸酯到烷基溴,从而在berkeley缩醛D中构建2,3-二氢呋喃环。[3]树烯的化学选择性甲基化和半氢化,以及溶剂控制的非对映选择性环氧化。除了为这些密集拥挤的天然产物提供合成途径外,我们的研究还提供了在结构要求高的分子组装中实现选择性的机制见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
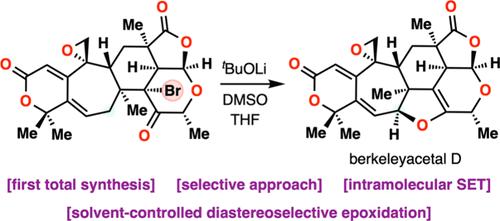
Total Synthesis of DMOA-Derived Meroterpenoids: Achieving Selectivity in the Synthesis of (+)-Berkeleyacetal D and (+)-Peniciacetal I
The synthesis of complex natural products requires efficient control over chemoselectivity, stereoselectivity, and regioselectivity. Berkeleyacetals, a subfamily of 3,5-dimethylorsellinic acid (DMOA)-derived meroterpenoids, pose substantial synthetic challenges due to their densely functionalized and highly oxidized architectures, which have constrained synthetic efforts. Here, we present the first total synthesis of this class of DMOA-derived meroterpenoids, specifically (+)-berkeleyacetal D and (+)-peniciacetal I. Our approach features a chemoselective deprotonation followed by an intramolecular single-electron transfer (SET) from an enolate to an alkyl bromide, enabling the construction of the 2,3-dihydrofuran ring in berkeleyacetal D. Additional selective transformations include an endo-selective intramolecular Diels–Alder reaction, chemoselective methylations and semihydrogenation of [3]dendralene, and a solvent-controlled diastereoselective epoxidation. Beyond providing a synthetic route to these densely congested natural products, our study offers mechanistic insights into achieving selectivity in the assembly of architecturally demanding molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


