类黄嘌呤A的不对称全合成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
首次在不使用保护基团的情况下,用14步完成了类janthinoid A的不对称全合成。通过环氧化物引发的阳离子π-环化反应和Fe(ClO4)3介导的氧化级联环化反应,分别构建了反式十烷亚基和刚性氧杂环[3.2.1]辛烷基序。本文章由计算机程序翻译,如有差异,请以英文原文为准。
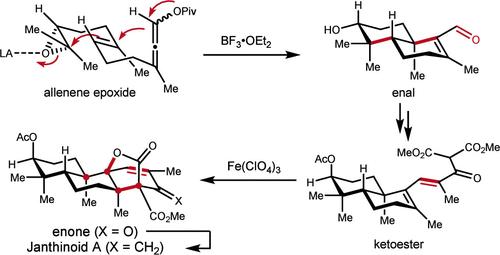
Asymmetric Total Synthesis of Janthinoid A
The asymmetric total synthesis of janthinoid A has been accomplished for the first time in 14 steps without using a protecting group. The trans-decalin subunit and the rigid oxabicyclo[3.2.1]octane motif were constructed via an epoxide-initiated cationic π-cyclization reaction and a Fe(ClO4)3-mediated oxidative cascade cyclization reaction, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


