表面羟基在甲酸在Fe3O4上脱水脱氢中的作用(001)
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
了解表面结构和羟基化在金属氧化物表面催化反应中的作用,对于深入了解复杂界面过程的机理非常重要。本文采用x射线光电子能谱、红外反射吸收能谱、程序升温反应能谱、低能电子衍射和电子结构计算相结合的方法研究了甲酸在重构Fe3O4(001)上的反应活性。我们发现甲酸最初在低温(< 80k)下解离成双齿甲酸和羟基,初始剂量为每个Fe3O4(001)单元电池覆盖两个HCOOH。在较高温度下(>450 K),甲酸盐主要沿着脱水途径分解,产生CO和H2O,脱氢成CO2是少数副反应。作为第一步,水的形成通过火星-范克雷文机制导致地表氧气的提取。计算研究表明,嵌入氧空位的甲酸盐是CO形成机制的关键中间体。CO的生成通过两种反应途径进行,其中解吸在530 K时在富羟基表面达到峰值,在560 K时在缺羟基表面达到峰值。原子氢共吸附实验和从头计算表明,表面羟基的存在降低了CO的形成屏障。这些结果突出了在金属氧化物表面的反应中,底物和中间物质之间复杂的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
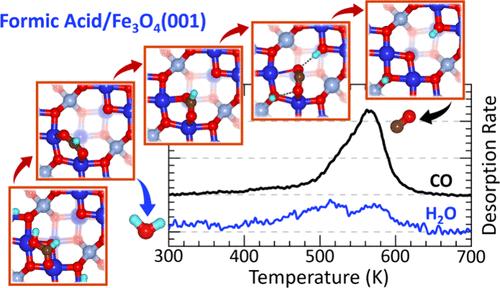
The Role of Surface Hydroxyls in Dehydration and Dehydrogenation of Formic Acid on Fe3O4(001)
Understanding the role of surface structure and hydroxylation in catalytic reactions on metal oxide surfaces is important for developing a mechanistic insight into the complex interface processes. Here, we investigate the reactivity of formic acid on reconstructed Fe3O4(001) using a combination of X-ray photoelectron spectroscopy, infrared reflection absorption spectroscopy, temperature-programmed reaction spectroscopy, low energy electron diffraction, and electronic structure calculations. We find that formic acid initially dissociates at low temperatures (<80 K) into bidentate formate and a hydroxyl up to an initial dosed coverage of two HCOOH per Fe3O4(001) unit cell. At higher temperatures (>450 K), formate largely decomposes along the dehydration pathway, producing CO and H2O, with dehydrogenation to CO2 being a minority side reaction. As a first step, water formation leads to surface oxygen extraction via the Mars-van Krevelen mechanism. Computational studies reveal formate embedded in oxygen vacancies as a key intermediate in the CO formation mechanism. CO formation proceeds via two reaction pathways with desorption that peaks at 530 K on the hydroxyl-rich surface and 560 K on the hydroxyl-deficient surface. Atomic hydrogen coadsorption experiments and ab initio calculations reveal that the presence of surface hydroxyls reduces the CO formation barrier. These results highlight the complex interactions between substrate and intermediate species occurring during reactions on metal oxide surfaces.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


