配体调节的无膦Ni(II)配合物用于炔的z选择性半转移加氢和氨硼烷对α,β-不饱和酮的双重转移加氢
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d4qo02326a
引用次数: 0
摘要
由于顺式/反式异构化和过度还原等问题,炔烃的立体选择性半转移加氢具有很高的挑战性。同样,在α,β-不饱和酮中同时实现C=O和C=C键的氢化也面临着巨大的挑战。合成了一系列含苯并咪唑基三齿和四齿配体的无膦Ni(II)、Mn(II)和Co(II)基配合物。其中,镍配合物在炔的z选择性半转移加氢反应和氨硼烷(AB)对查尔酮的双转移加氢反应中表现出优异的反应活性。基于配体和催化剂的设计,提出了选择性和高效的转移加氢方案。Cat. 4具有较大的配体,促进了炔的z选择性半转移加氢,而Cat. 1具有相对较小的配体,使α,β-不饱和酮双转移加氢为饱和醇。采用温和、无添加剂的工艺,顺利地得到了多种具有合成价值的z -烯烃和功能化醇。几个控制实验,以及DFT计算提供了催化循环的关键见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
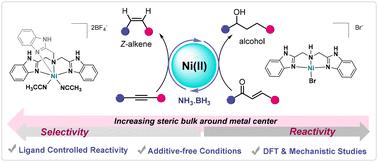
Ligand-modulated phosphine-free Ni(ii) complexes for Z-selective semi-transfer hydrogenation of alkynes and dual transfer hydrogenation of α,β-unsaturated ketones using ammonia borane†
Stereoselective semi-transfer hydrogenation of alkynes is highly challenging due to issues with cis/trans-isomerization and over-reduction. Similarly, achieving simultaneous hydrogenation of both CO and CC bonds in α,β-unsaturated ketones poses substantial challenges. A series of phosphine-free Ni(ii), Mn(ii), and Co(ii)-based complexes bearing benzimidazole-based tri- and tetradentate ligands were synthesized. Among them, the nickel complexes exhibited superior reactivity in the Z-selective semi-transfer hydrogenation of alkynes and the dual transfer hydrogenation of chalcones using ammonia borane (AB). Selective and efficient transfer hydrogenation protocols were developed based on strategic ligand and catalyst design. Cat. , having a bulkier ligand, facilitated the Z-selective semi-transfer hydrogenation of alkynes, while Cat. , with a relatively less bulky ligand, enabled the dual transfer hydrogenation of α,β-unsaturated ketones to saturated alcohols. Employing these mild, additive-free protocols, a broad range of synthetically valuable Z-alkenes and functionalized alcohols were smoothly obtained. Several control experiments, along with DFT calculations, provided critical insights into the catalytic cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


