羧酸的直接C1同源化:由吖啶催化实现的自由基方法
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d4qo02442g
引用次数: 0
摘要
描述了羧酸的光催化C1同源化。转换是在一个方便的单锅过程中完成的。该反应的关键步骤是在双吖啶/癸烯酸光催化体系下,在乙基乙醛酸甲酰腙上进行脱羧自由基加成反应。所得到的肼中间体通过碱处理很容易转化为同源羧酸酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
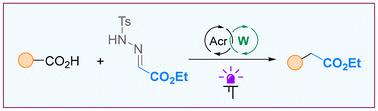
Direct C1 homologation of carboxylic acids: free radical approach enabled by acridine catalysis†
A photocatalytic C1 homologation of carboxylic acids is described. The transformation is performed in a convenient one-pot procedure. The key step is decarboxylative radical addition to a tosylhydrazone derived from ethyl glyoxylate, which is mediated by a dual acridine/decatungstate photocatalytic system. The resulting hydrazide intermediate is easily converted to the homologated carboxylic ester by treatment with a base.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


