可见光光氧化还原催化下后期N-N键形成的双夏霉素A和B的全合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
分子间氧化N-N键形成反应是相当具有挑战性的,很大程度上是未知的。N-N键二聚体吲哚半萜生物碱是一类未被开发的天然产物,直接脱氢形成N-N键的策略是有限的。在这里,我们报道了一个后期的可见光光氧化还原催化促进了N-N键的形成,导致阿肯色非对映体dixiamycin a (1a)和B (1b)的完全合成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
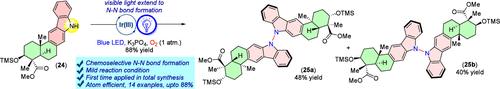
Total Synthesis of Dixiamycins A and B via a Late-Stage N-N Bond Formation under Visible Light Photoredox Catalysis.
Intermolecular oxidative N-N bond formation reactions are quite challenging and are largely uncharted. N-N linked dimeric indolosesquiterpene alkaloids represent an underexplored class of natural products, and strategies for direct dehydrogenative N-N bond formation are limited. Here, we have reported that a late-stage visible-light photoredox catalysis facilitates N-N bond formation, leading to the total syntheses of atropo-diastereomers dixiamycins A (1a) and B (1b).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


