预防使用PEG-rhG-CSF对晚期NSCLC一线免疫化疗的影响:一项队列研究
IF 3.5
Q2 ONCOLOGY
引用次数: 0
摘要
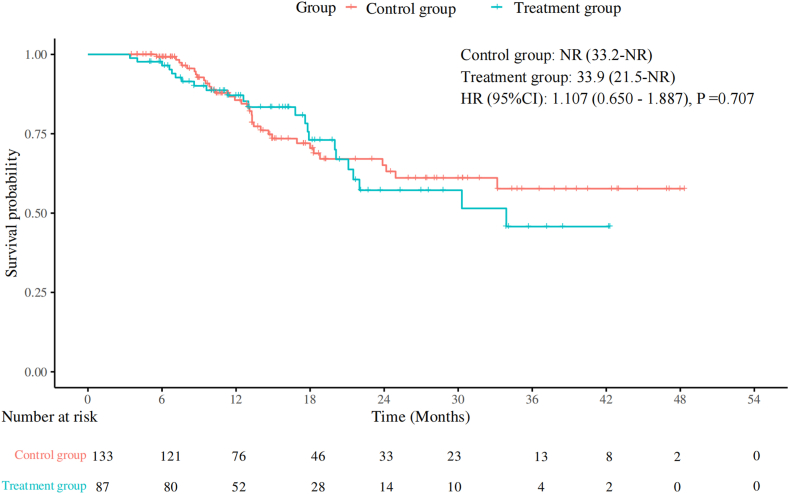
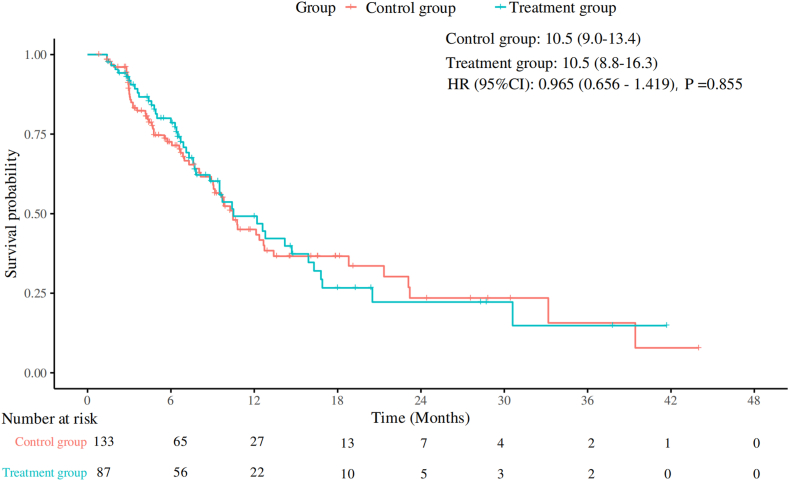
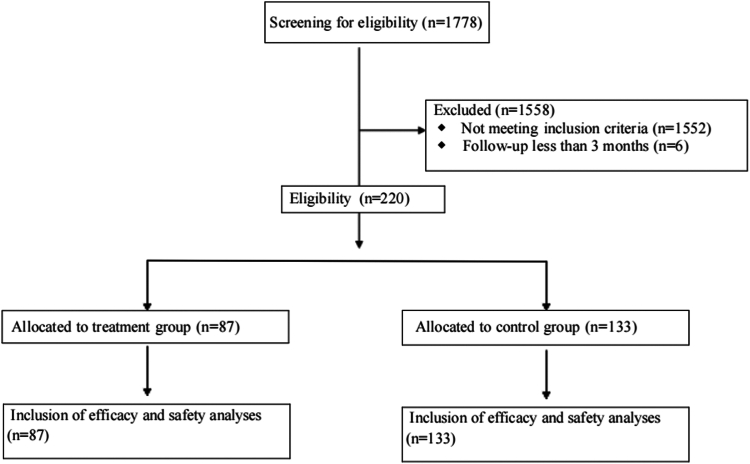
本研究旨在评估预防使用PEG-rhG-CSF对晚期NSCLC一线免疫化疗的影响。方法:选取2019年1月至2024年7月在中国医科大学盛京医院接受一线免疫化疗的晚期非小细胞肺癌患者为研究对象。患者分为以下两组:治疗组在免疫化疗后48小时接受预防性PEG-rhG-CSF(≥1个周期),对照组不接受PEG-rhG-CSF。主要终点为无进展生存期(PFS)、总生存期(OS)、总缓解率和安全性。进行倾向评分匹配分析以减少潜在的混杂因素。结果:共纳入220例患者,其中治疗组87例,对照组133例。治疗组和对照组的中位PFS均为10.5个月(p = 0.86),治疗组的中位OS为33.9个月,而对照组未达到OS (p = 0.71)。治疗组总有效率为64.4%,对照组为58.6% (p = 0.40)。经过倾向评分匹配分析(每组包括78例患者),治疗组的中位PFS为12.6个月,而对照组为10.5个月(p = 0.99),治疗组的中位OS为30.3个月,而对照组未达到(p = 0.85)。治疗组化疗中断、任何级别的白细胞减少、任何级别的中性粒细胞减少以及3至5级中性粒细胞减少的发生率均有所降低,且免疫相关不良事件未增加。结论:在接受一线免疫化疗的晚期非小细胞肺癌患者中预防性使用PEG-rhG-CSF不会影响疗效和安全性。它减少了化疗中断和中性粒细胞减少,而不增加免疫相关不良事件,从而支持安全和不间断的治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of Prophylactic Use of PEG-rhG-CSF on First-Line Immunochemotherapy in Advanced NSCLC: A Cohort Study
Introduction
This study aimed to assess the impact of prophylactic use of PEG-rhG-CSF on first-line immunochemotherapy in advanced NSCLC.
Methods
A cohort of patients with advanced NSCLC who received first-line immunochemotherapy at Shengjing Hospital of China Medical University between January 2019 and July 2024 was selected for this study. Patients were divided into the following two groups: a treatment group that received prophylactic PEG-rhG-CSF (≥1 cycle) 48 hours after immunochemotherapy and a control group that did not receive PEG-rhG-CSF. The primary end points were progression-free survival (PFS), overall survival (OS), overall response rate, and safety. A propensity score-matched analysis was performed to reduce potential confounders.
Results
A total of 220 patients were enrolled, with 87 in the treatment group and 133 in the control group. Median PFS was 10.5 months in both the treatment and control groups (p = 0.86), and median OS was 33.9 months in the treatment group versus not reached in the control group (p = 0.71). The overall response rate was 64.4% in the treatment group and 58.6% in the control group (p = 0.40). After propensity score-matched analysis (each group included 78 patients), median PFS was 12.6 months in the treatment group versus 10.5 months in the control group (p = 0.99), and median OS remained 30.3 months in the treatment group versus not reached in the control group (p = 0.85). The treatment group had a reduced incidence of chemotherapy interruptions, any grade of leukopenia, any grade of neutropenia, and grades 3 to 5 neutropenia, without an increase in immune-related adverse events.
Conclusions
The prophylactic use of PEG-rhG-CSF in patients with advanced NSCLC undergoing first-line immunochemotherapy did not compromise efficacy and safety. It reduced chemotherapy interruptions and neutropenia, without increasing immune-related adverse events, thus supporting safe and uninterrupted treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JTO Clinical and Research Reports
Medicine-Oncology
CiteScore
4.20
自引率
0.00%
发文量
145
审稿时长
19 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


