提高重组金(110)表面反应的化学选择性:以戊烯桥接聚二烯类似物为例
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
聚苯乙烯类似物由非苯环分隔的短苯乙烯片段组成,具有有趣的电子性质和磁相互作用。特别是戊二烯桥接聚烯(PPs),有望提高电导率和潜在的开壳基态。然而,由于溶解性差和合成方案有限,PPs在溶液化学中仍然难以捉摸。在这里,我们报道了通过邻二甲苯之间的环化在表面合成PPs。扫描隧道显微镜和原子力显微镜显示,重建的Au(110)表面明显增强了环化过程的化学选择性。扫描隧道光谱结合密度泛函理论表明,PP具有窄的直接带隙,类似于长碳烯。这项工作证明了通过结合非苯环在聚二烯类似物中进行带结构工程的潜力,为有机电子学和自旋电子学的进步铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
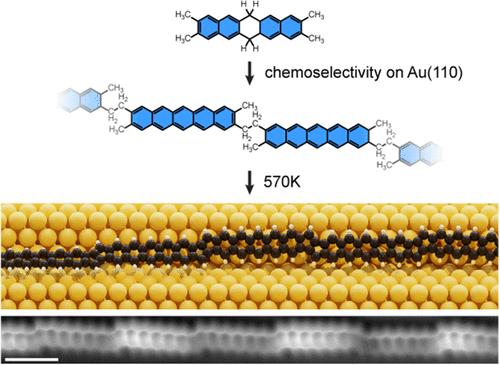
Enhancing Chemoselectivity of On-Surface Reactions on Reconstructed Au(110): The Case of a Pentalene-Bridged Polyacene Analogue
Polyacene analogues, consisting of short acene segments separated by nonbenzenoid rings, offer intriguing electronic properties and magnetic interactions. Pentalene-bridged polyacenes (PPs), in particular, hold promise for enhancing the electrical conductivity and potential open-shell ground states. However, PPs have remained elusive in solution chemistry due to poor solubility and limited synthetic protocols. Here, we report the on-surface synthesis of PPs through the annulation between ortho-xylene groups. Scanning tunneling microscopy and atomic force microscopy reveal that the reconstructed Au(110) surface significantly enhances the chemoselectivity of the annulation process. Scanning tunneling spectroscopy combined with density functional theory suggests that PP exhibits a narrow direct band gap, similar to long acenes. This work demonstrates the potential for band structure engineering in polyacene analogues by incorporating nonbenzenoid rings, paving the way to advancements in organic electronics and spintronics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


