H2O2 在溶热合成绿色发光氮掺杂石墨烯量子点中的多重作用
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
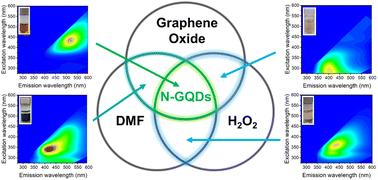
Multifaceted role of H2O2 in the solvothermal synthesis of green-emitting nitrogen-doped graphene quantum dots
Fluorescent nitrogen-doped carbon dots (N-GQDs) with long-wavelength emission properties are of increased interest for technological applications. They are widely synthesized through the solvothermal treatment of graphene oxide (GO) using N,N-dimethylformamide (DMF) as a cleaving and doping agent. However, this process simultaneously generates undesired interfering blue-emissive by-products. In this study, we present a straightforward method for synthesizing N-GQDs exclusively exhibiting green fluorescence. The key innovation lies in the addition of hydrogen peroxide (H2O2) to the DMF-driven one-pot solvothermal cleavage process. Systematically controlling the reaction conditions, we elucidate the threefold beneficial role of H2O2: first, it acts as a radical source facilitating the degradation of DMF and the generation of nitrogen-containing radicals, essential for N-GQD formation; second, it prevents the thermal reduction of GO, thus ensuring persistent reaction pathways with DMF-derived radicals; and third, it suppresses the self-reaction of DMF-derived radicals, thereby avoiding the formation of undesired blue-fluorescent by-products. Our findings on the reaction mechanism and the advantageous role of H2O2 open new possibilities for the rational design of N-GQDs genuinely emitting at long wavelengths.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


