trbf4催化氟化物回收实现分子内氟酰化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
炔系和烯烃系酰基氟化物通过TrBF4催化氟回收进行分子内碳氟化。炔加成物具有优异的立体选择性,可获得新型氟化茚-2-酮(均≥95:5 E/Z)和环戊烷-2-酮(85:15 E/Z)。氟化铬-2- 1和叔烷基氟化物也可以用这种方法合成,与以前报道的在更苛刻的条件下使用昂贵的金属催化剂的方案相比,这是有利的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
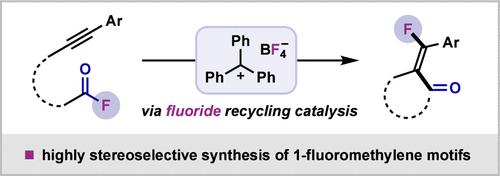
Intramolecular Fluoroacylation Enabled by TrBF4-Catalyzed Fluoride Recycling
Alkyne- and alkene-tethered acyl fluorides undergo intramolecular carbofluorination via fluoride recycling using catalytic TrBF4. Excellent stereoselectivity is observed for the alkyne addition, enabling access to novel fluorinated indan-2-ones (all ≥95:5 E/Z) and cyclopentan-2-ones (85:15 E/Z). Fluorinated chroman-2-ones and tertiary alkyl fluorides can also be synthesized using this method, comparing favorably to previously reported protocols that employ expensive metal catalysts under harsher conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


