三氟化硅基促进磺化
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
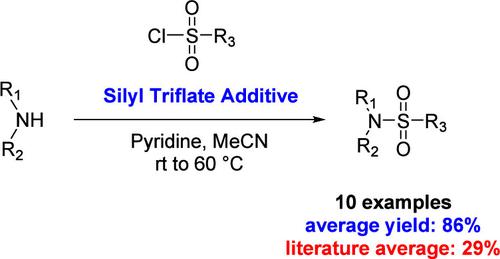
磺胺类是天然产品和医药产品中普遍存在的官能团。当使用亲核胺和亲电性磺酰氯时,磺胺类化合物的合成通常很简单。当非传统基质出现反应性挑战时,可能需要苛刻的条件或新的合成路线。在这里,我们报告了一种使用三氟化硅酯添加剂来克服反应性限制的方法,当电子缺乏和空间阻碍胺与电子丰富或体积庞大的磺酰氯一起使用时。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Silyl Triflate-Promoted Sulfonylations
Sulfonamides are prevalent functional groups represented in both natural and pharmaceutical products. The synthesis of sulfonamides is often straightforward when using nucleophilic amines and electrophilic sulfonyl chlorides. When reactivity challenges arise for nontraditional substrates, harsh conditions or new synthetic routes may be required. Here we report a method using silyl triflate additives to overcome reactivity limitations when electron deficient and sterically encumbered amines are used in conjunction with electron rich or bulky sulfonyl chlorides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


