阴离子调制溶剂化鞘和电双层使锂离子存储从- 60至80°C
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
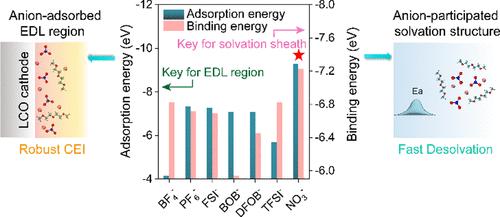
目前的锂电池在极端温度条件下,无论是高温还是低温,性能都会显著下降。传统的宽温度电解质设计通常通过操纵溶剂化鞘和选择具有极端熔点/沸点的溶剂来解决这些挑战。然而,这些溶剂介导的溶液,虽然在一个极端温度下有效,但由于在宽温度下保持溶剂稳定性的固有困难,总是在另一端失败。在此,我们报告了使用主锂盐同时解决极高和低温下的界面挑战。这种方法不同于传统的溶剂介导的策略。作为概念验证,我们利用硝酸锂(LiNO3)建立了阴离子控制的溶剂化结构和双电层。配制的电解质在极端温度下表现出卓越的性能,在- 60°C下保持56.1%的容量,在80°C下保持400次稳定循环。相比之下,基于当前溶剂介导策略的电解质不能在- 60°C下工作,不能在80°C下超过50个循环。通过将重点转移到主盐而不是溶剂上,我们的工作为解决电解质在广泛温度范围内稳定性的长期挑战提供了可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Anion-Modulated Solvation Sheath and Electric Double Layer Enabling Lithium-Ion Storage From −60 to 80 °C
Current lithium batteries experience significant performance degradation under extreme temperature conditions, both high and low. Traditional wide-temperature electrolyte designs typically addressed these challenges by manipulating the solvation sheath and selecting solvents with extreme melting/boiling points. However, these solvent-mediated solutions, while effective at one temperature extreme, invariably fail at the opposite end due to the inherent difficulties in maintaining solvent stability across wide temperatures. Herein, we report the use of the main lithium salt to simultaneously address interfacial challenges at both extremely high and low temperatures. This approach is different from the conventional solvent-mediated strategies. As a proof of concept, we utilized lithium nitrate (LiNO3) to establish an anion-controlled solvation structure and electric double layer. The formulated electrolytes exhibited remarkable performance across temperature extremes, retaining 56.1% capacity at −60 °C and sustaining 400 stable cycles at 80 °C. In contrast, electrolytes based on current solvent-mediated strategies failed to operate at −60 °C and could not exceed 50 cycles at 80 °C. By shifting the focus to the main salt rather than the solvent, our work offers the possibility of addressing the enduring challenges of electrolyte stability across a broad temperature range.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


