金(I)-通过1,2-烷基/芳基硫离子迁移催化2-脱氧-β-糖基化:丝瓜苷A五糖的合成
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
2-脱氧-β-糖苷是天然产物和药物的重要成分;然而,相应的2-脱氧-β-糖苷键的化学构造具有挑战性。本文描述了一种高效的催化方案,通过ipraunt_2催化激活独特的1,2-反式位置的c2 - s-丙基黄药(OSPX)离开基,或(PhO) 3paunt_2催化激活相应巯基苷的1,2-反式- c2 -邻炔基苯甲酸酯(OABz)取代基来合成2-脱氧-β-糖苷。这些活化过程触发1,2-烷基/芳基硫代迁移糖基化,使在温和的反应条件下合成结构多样的2-脱氧-β-糖苷。这一策略的力量通过首次合成与velutinoside A对应的五糖链得到了证明,其特征是金(I)催化构建了四个连续的β-l-oleandrosidic键,以会聚和一锅糖基化的方式。机制研究,包括对照实验和氘标记实验,强调OSPX的关键作用和金(I)活化的C≡C三键在糖基化过程中的参与。低温核磁共振实验揭示了金(I)催化剂与OSPX的硫羰基和炔基的独特双配位模式,引发了5-外显式环化过程。此外,密度泛函理论(DFT)模拟揭示了配体诱导的离去基OSPX和OABz与金催化剂IPrAuNTf2和(PhO)3PAuNTf2之间的匹配不匹配效应。DFT模拟还表明,2-脱氧-β-糖苷键的形成是通过受体对氧羰基中间体的底面攻击而发生的,氧羰基中间体采用4H3半椅构象,导致能量有利的4c1构象中间体Dβ通过氢键相互作用稳定。本文章由计算机程序翻译,如有差异,请以英文原文为准。
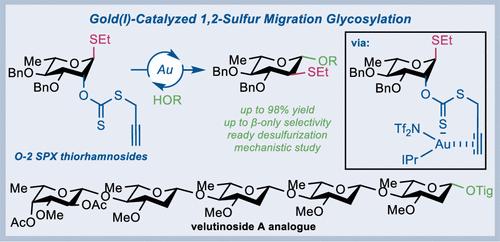
Gold(I)-Catalyzed 2-Deoxy-β-glycosylation via 1,2-Alkyl/Arylthio Migration: Synthesis of Velutinoside A Pentasaccharide
2-Deoxy-β-glycosides are essential components of natural products and pharmaceuticals; however, the corresponding 2-deoxy-β-glycosidic bonds are challenging to chemically construct. Herein, we describe an efficient catalytic protocol for synthesizing 2-deoxy-β-glycosides via either IPrAuNTf2-catalyzed activation of a unique 1,2-trans-positioned C2-S-propargyl xanthate (OSPX) leaving group or (PhO)3PAuNTf2-catalyzed activation of a 1,2-trans-C2-ortho-alkynylbenzoate (OABz) substituent of the corresponding thioglycosides. These activation processes trigger 1,2-alkyl/arylthio-migration glycosylation, enabling the synthesis of structurally diverse 2-deoxy-β-glycosides under mild reaction conditions. The power of this strategy is demonstrated by the first synthesis of the pentasaccharide chain corresponding to velutinoside A, which features gold(I)-catalyzed construction of four successive β-l-oleandrosidic bonds in both a convergent and a one-pot glycosylation manner. Mechanistic studies, including control experiments and deuterium-labeling experiments, emphasize the crucial role of the OSPX and the involvement of the gold(I)-activated C≡C triple bond during the glycosylation process. The low-temperature NMR experiments unveiled a unique dual-coordination pattern of the gold(I) catalyst to the thiocarbonyl group and the alkynyl group of the OSPX, initiating a 5-exo-dig cyclization process. Furthermore, density functional theory (DFT) simulations reveal the ligand-induced match-mismatch effect between leaving groups OSPX and OABz and gold catalysts IPrAuNTf2 and (PhO)3PAuNTf2. The DFT simulations also suggest that the formation of 2-deoxy-β-glycosidic bonds occurs via the bottom-face attack of the acceptor to the oxocarbenium intermediate, which adopts a 4H3 half-chair conformation, leading to an energetically favored, 4C1-conformed intermediate Dβ that is stabilized by a hydrogen bonding interaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


