协同镍/锌催化催化丁-3-烯酯的形式酯交换反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
过渡金属催化的偶联反应更新了酯作为亲电伙伴的作用。在这种情况下,我们描述了一个协同Ni/ zn催化的丁-3-烯基酯与四氢呋喃和碘化烷基的形式酯交换反应,得到4-烷氧基丁基酯。芳香族酯和脂肪族酯都是亲电试剂,从而扩大了酯在偶联反应中的底物范围,因为以前报道的亲电试剂严格限于芳香族酯。机理研究表明,在镍的催化作用下,酯类的C(酰基)-O键甚至更惰性的C(烷基)-O键都可以断裂。提出了两种催化循环,代表了一种相对于传统酯交换反应的新反应途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
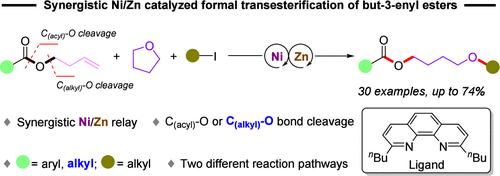
Formal Transesterification Reaction of But-3-enyl Esters Enabled by a Synergistic Nickel/Zinc Relay
The transition metal-catalyzed coupling reaction has renewed the role of ester as an electrophilic partner. In this context, we describe a synergistic Ni/Zn-catalyzed formal transesterification reaction of but-3-enyl esters with tetrahydrofuran and alkyl iodides to give 4-alkoxylbutyl esters. The aromatic and aliphatic esters are both competent electrophiles and thus broaden the substrate scope of esters in coupling reactions, because the electrophiles in previously reported work were strictly limited to aromatic ones. Mechanistic studies reveal that the C(acyl)–O bond or even more inert C(alkyl)–O bond of esters could be cleaved under nickel catalysis. Two catalytic cycles are proposed, which represent a new reaction pathway versus the traditional transesterification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


