镍催化炔的三组分碳胺化/环化制备2,3-二取代喹啉
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文提出了一种镍催化的化学和区域选择性三组分串联碳胺化和末端炔与有机硼酸和邻苯胺环化,以方便和模块化地获得2,3-取代喹啉。在这个过程中,蒽醌具有双重作用:作为亲电胺化试剂和氧化还原缓冲剂,抑制非循环Ni(0)络合物的生成。此外,阴离子乙酰丙酮酸(acac)配体对于确保高效的Ni(I) -Ni (III) -Ni (I)催化循环至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
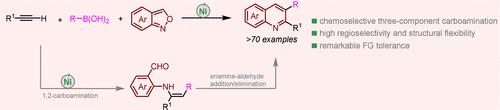
Nickel-Catalyzed Three-Component Carboamination/Cyclization of Alkynes To Access 2,3-Disubstituted Quinolines
Presented herein is a nickel-catalyzed chemo- and regioselective three-component tandem carboamination and cyclization of terminal alkynes with organoboronic acids and anthranils for facile and modular access to 2,3-substituted quinolines. In this process, anthranil has dual roles: serving as an electrophilic aminating reagent and a redox buffer to suppress the generation of an off-cycle Ni(0) complex. Moreover, the anionic acetylacetonate (acac) ligand was found to be vital to ensure a productive Ni(I)–Ni(III)–Ni(I) catalytic cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


