阳离子对电化学铂溶解的影响
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
保证电催化剂的稳定性对电化学能量转换装置的成功至关重要。降解是一个基本的过程,涉及到带正电的金属离子释放到电双层(EDL)中,并随后扩散到大块电解质中。然而,尽管它对实现长时间电催化至关重要,催化剂溶解与EDL结构的潜在因果关系在很大程度上仍然未知。在这里,我们发现Pt的电化学溶解受到电解液中碱金属阳离子(AM+)的强烈影响。通过实时监测铂溶出情况,发现Li+ >序列中铂的浸出有减少的趋势;Na +比;K +比;c +。我们的计算预测表明,界面OH -浓度在Pt溶解中起着关键作用,其中OH -通过中和Ptz+物质,促进溶解的Pt离子向外扩散到体电解质中,从而筛选其再沉积的迁移力。结合这一理论结果,我们验证了溶解Pt的量与AM+的水解pKa或酸度之间存在很强的相关性,表明AM+的特性决定了局部OH -浓度,从而改变了Pt的溶解量。我们的研究结果强调了调整EDL结构以实现持久电催化的必要性,这是未来研究的一个有前途的领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
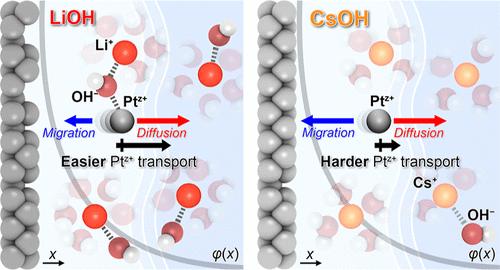
Cation Effect on the Electrochemical Platinum Dissolution
Ensuring the stability of electrocatalysts is paramount to the success of electrochemical energy conversion devices. Degradation is a fundamental process involving the release of positively charged metal ions into the electric double layer (EDL) and their subsequent diffusion into the bulk electrolyte. However, despite its vital importance in achieving prolonged electrocatalysis, the underlying causality of catalyst dissolution with the EDL structure remains largely unknown. Here, we show that electrochemical Pt dissolution is strongly influenced by the identity of the alkali metal cation (AM+) in the electrolyte. By monitoring Pt dissolution in real-time, we found a trend of reduced Pt leaching in the sequence Li+ > Na+ > K+ > Cs+. Our computational predictions suggest that interfacial OH– concentration plays a pivotal role in Pt dissolution, where OH– facilitates the outward diffusion of dissolved Pt ions into the bulk electrolyte by neutralizing the Ptz+ species, thereby screening the migration force for their redeposition. Combined with this theoretical result, we verify a strong correlation between the amount of dissolved Pt and the hydrolysis pKa or acidity of AM+, indicating that the AM+ identity determines the local OH– concentration and thereby modifies the amount of Pt dissolution. Our results underscore the need to tune the EDL structure to achieve durable electrocatalysis, a promising area for future research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


