从自由基偶联到对映选择性控制质子化:推进立体中心的精确构建
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
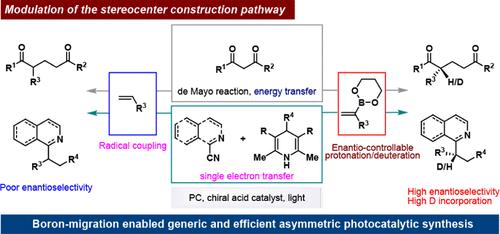
绿色和可持续平台的最新进展,特别是可见光驱动的光催化,促进了自由基化学的重大进展,使在温和条件下从简单易得的原料高效合成重要分子成为可能。然而,自由基的快速轨道翻转和高反应性为通过自由基耦合实现立体中心形成的精确对映体控制带来了巨大挑战。在这项研究中,我们提出了一种通用而有效的策略来调节这种难以捉摸的方法,通过1,3-硼迁移促进对映体可控质子化。我们成功地开发了两种以前难以捉摸的光催化不对称转化:利用能量转移的de Mayo反应和由单电子转移引发的氰氮杂芳烃三组分反应。此外,加入具有成本效益的D2O作为氘源,增强了该方法的合成和制药意义,为未来的应用提供了有价值的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
From Radical Coupling to Enantioselective Controlled Protonation: Advancing Precise Construction of Stereocenters
Recent advancements in green and sustainable platforms, particularly visible light-driven photocatalysis, have spurred significant progress in radical chemistry, enabling the efficient synthesis of important molecules from simple and readily available feedstocks under mild conditions. However, the rapid orbital flipping and high reactivity of radicals pose substantial challenges for achieving precise enantiocontrol in stereocenter formation via radical coupling. In this study, we present a generic and efficient strategy that modulates this elusive approach, facilitating enantiocontrollable protonation through 1,3-boron migration. We successfully developed two previously elusive photocatalytic asymmetric transformations: the de Mayo reaction utilizing energy transfer and three-component reactions of cyanoazaarenes initiated by single-electron transfer. Moreover, the incorporation of cost-effective D2O as a deuterium source enhances the synthetic and pharmaceutical significance of this method, offering a valuable tool for future applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


