钯催化氧化烯-烯交叉偶联
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
两个相似的未激活伙伴之间的直接交叉偶联反应具有挑战性,但却构成了有机合成中创造新的碳-碳键的有力策略。[4]树突烯是一类具有快速生成结构复杂性的无环支链共轭寡烯,具有很大的合成潜力,但由于其可及性有限,其化学性质仍是一个未开发的领域。在此,我们报道了一种高选择性钯催化的两个烯烯的氧化交叉偶联,其中一个烯烯中存在定向烯烃,从而可以以会聚模块化的方式轻松合成广泛的功能化[4]枝烯。具体来说,烯丙基取代基作为辅助基团的烯丙烯的选择性C-H活化产生乙烯基钯中间体,该中间体在碳置换中与取代较少的烯丙烯反应,随后是β-氢化物消除。反应顺序导致两个二烯单元之间形成新的C(sp2) -C (sp2)键。值得注意的是,该方案为C(乙烯基)-C(乙烯基)键的位点选择性和立体选择性构建提供了一种非常规的策略,而不使用任何卤化和有机金属烯烃前体。此外,合成的[4]树突烯的实际转化和生物相关分子的后期修饰证明了它们在天然产物的全合成和药物发现方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
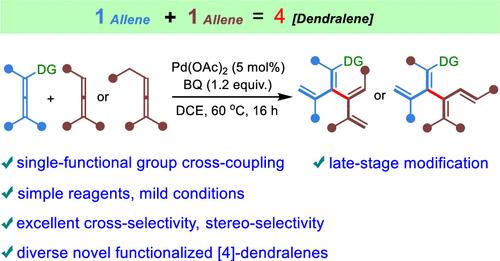
Palladium-Catalyzed Oxidative Allene–Allene Cross-Coupling
Direct cross-coupling reactions between two similar unactivated partners are challenging but constitute a powerful strategy for the creation of new carbon–carbon bonds in organic synthesis. [4]Dendralenes are a class of acyclic branched conjugated oligoenes with great synthetic potential for the rapid generation of structural complexity, yet the chemistry of [4]dendralenes remains an unexplored field due to their limited accessibility. Herein, we report a highly selective palladium-catalyzed oxidative cross-coupling of two allenes with the presence of a directing olefin in one of the allenes, enabling the facile synthesis of a broad range of functionalized [4]dendralenes in a convergent modular manner. Specifically, the selective allenic C–H activation of an allene with an allyl substituent as the assisting group gives rise to a vinylpalladium intermediate, which reacts with a less substituted allene in a carbopalladation, followed by a β-hydride elimination. The reaction sequence leads to a new C(sp2)–C(sp2) bond between two diene units. Remarkably, this protocol provides an unconventional strategy for the site-selective and stereoselective construction of C(vinyl)–C(vinyl) bonds without using any halogenated and organometallics olefin precursors. Furthermore, the practical transformations of the synthesized [4]dendralenes and late-stage modifications of biorelevant molecules demonstrate their potential in the total synthesis of natural products and drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


