镍催化邻碳硼烷区域选择性B-H多硫化的可变双齿无迹导向基团
IF 6.1
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在无氧化剂和弱碱性条件下,利用可变双齿8-氨基喹啉无踪导向基团(TDG),制备了镍催化的二十面体碳硼烷簇的直接笼型B-H多硫化反应,得到了用于硼中子俘获治疗(BNCT)的硼递送剂中的多硫化/硒化邻碳硼烷。通过简单的底物结构修饰(邻碳硼烷取代c2),实现了对tdg的可控选择性去除和保留。广泛的二硫化物、二硒化物、噻吩和苯烯醇,以及邻碳硼烷底物,是相容的,产生不同功能化的邻碳硼烷与四碳和三碳取代。目前的方案为通过可变双齿TDG策略进行无氧化剂和无强碱、贱金属催化的碳硼烷的区域选择性迭代B-H加氢反应开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
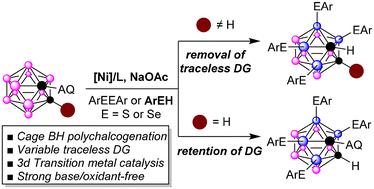
A variable bidentate traceless directing group for nickel-catalyzed regioselective B–H poly-chalcogenation of o-carboranes
A nickel-catalyzed direct cage B–H poly-chalcogenation of icosahedral carborane clusters, enabled by a variable bidentate 8-aminoquinoline traceless directing group (TDG), has been developed under oxidant-free and weakly basic conditions, resulting in poly-thiolated/selenylated o-carboranes present in boron delivery agents for boron neutron capture therapy (BNCT). The controllable selectivity of removal and retention of these TDGs was achieved through simple substrate structure modification (C2-substitution in o-carboranes). A wide range of disulfides, diselenides, thiophenols and benzeneselenols, as well as o-carborane substrates, are compatible, generating diversely functionalized o-carboranes with tetra- and tri-chalcogen substitution. The current protocol opens new avenues for oxidant- and strong base-free, base-metal-catalyzed regioselective iterative B–H chalcogenation of carboranes via a variable bidentate TDG strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


