内吞系索调节非常规GAPDH分泌。
IF 6.2
Q1 Immunology and Microbiology
引用次数: 0
摘要
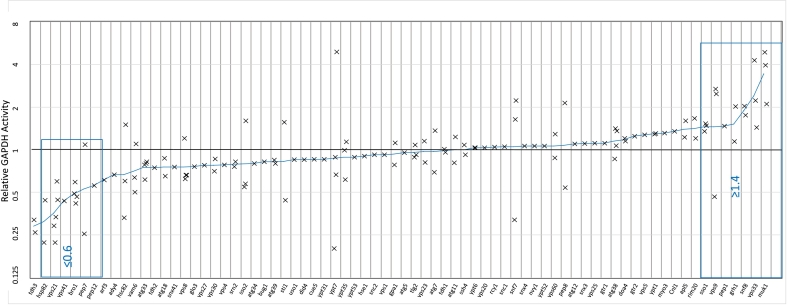
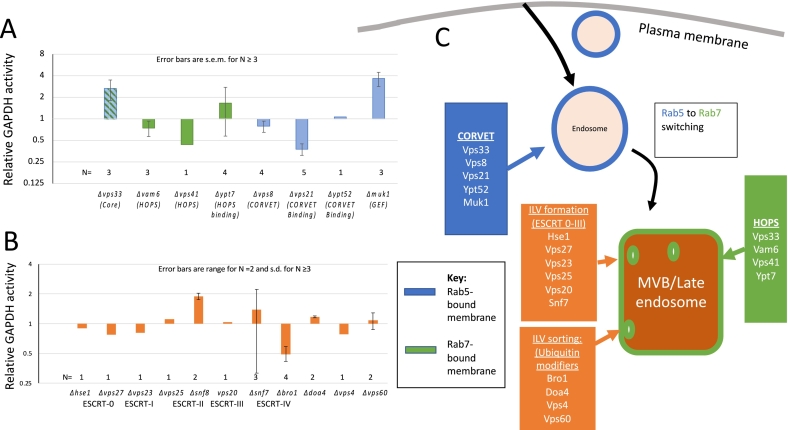
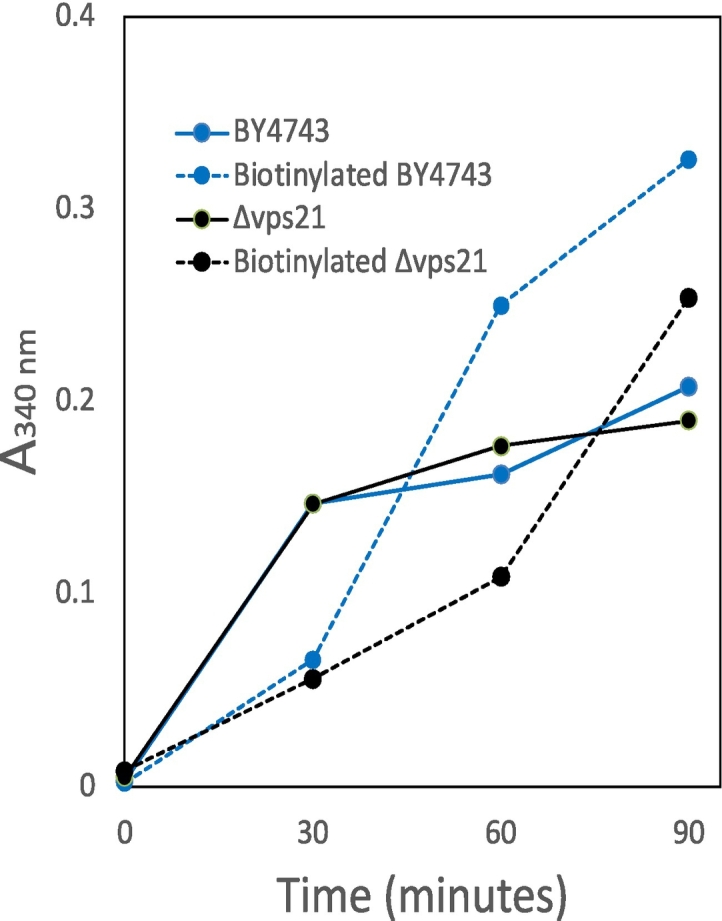
酵母细胞壁包含经典分泌和非常规分泌的蛋白质。后一类缺乏转运到内质网的信号序列,因此这些蛋白质通过未知的机制转运到壁。其中一种蛋白质是糖酵解酶甘油醛-3-磷酸脱氢酶(GAPDH),它丰富于细胞质中,但也存在于酵母菌细胞壁中,在那里它具有酶活性。我们筛选二倍体酿酒酵母纯合基因缺失的细胞壁GAPDH活性的变化。内溶酶体系统中针对内噬系链的缺失对GAPDH分泌的影响最大,包括vps21、bro1、vps41和pep12。主要的GAPDH亚型Tdh3部分定位于内溶酶体室室,包括多泡体,这是非常规蛋白质分泌途径的常见入口点。缺乏内体Rab5-GTPase Vps21的酵母在GAPDH分泌方面存在缺陷,并且延迟进入内溶酶体腔室。因此,我们得出结论,进入内溶酶体室有助于GAPDH的非常规分泌。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Endocytic tethers modulate unconventional GAPDH secretion
Yeast cell walls contain both classically-secreted and unconventionally-secreted proteins. The latter class lacks the signal sequence for translocation into the ER, therefore these proteins are transported to the wall by uncharacterized mechanisms. One such protein is the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) which is abundant in the cytosol, but also found in the yeast cell wall where it is enzymatically active. We screened diploid Saccharomyces cerevisiae homozygous gene deletions for changes in cell wall GAPDH activity. Deletions targeting endocytic tethers in the endolysosomal system had the largest effects on GAPDH secretion, including vps21, bro1, vps41, and pep12. The predominant GAPDH isoform Tdh3 was partially localized to endolysosomal compartments, including multivesicular bodies, which are common entry points to unconventional protein secretion pathways. Yeast lacking the endosomal Rab5-GTPase Vps21 had defects in GAPDH secretion as well as delayed entry into to the endolysosomal compartments. Therefore, we conclude that entry into the endolysosomal compartment facilitates non-conventional secretion of GAPDH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Surface
Immunology and Microbiology-Applied Microbiology and Biotechnology
CiteScore
6.10
自引率
0.00%
发文量
18
审稿时长
49 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


