alfa在日本迟发性和婴儿期庞贝病患者中的疗效和安全性:来自临床试验的病例系列
IF 1.9
4区 医学
Q3 GENETICS & HEREDITY
引用次数: 0
摘要
背景:avalglucosidase alfa治疗Pompe病(PD)的有效性和安全性已经在一项针对迟发性PD (LOPD)患者的全球3期试验(COMET)和一项针对婴儿期PD (IOPD)患者的全球2期试验(Mini-COMET)中得到证实。本病例系列研究了参加这些试验的三名日本患者的个体结果。方法:收集COMET和Mini-COMET试验中收集的病例报告。详细的方法以前已经报道过。COMET的主要终点是从基线到第49周的直立用力肺活量百分比(FVC %)预测的变化。Mini-COMET的主要终点是阿瓦洛葡萄糖苷酶α的安全性和耐受性。在两项试验中,关键的次要终点包括运动功能测试和其他定性改善措施。两项试验还评估了生物标志物和抗药物抗体的变化。结果:日本患者的结果代表了COMET和Mini-COMET试验中总体人群的结果。我们详细介绍了一名参加COMET试验的日本LOPD患者和两名参加Mini-COMET试验的日本IOPD患者的结果。重要的是,日本LOPD和IOPD患者分别在20mg /kg和40mg /kg剂量下,avalglucosidase alfa耐受性良好。结论:虽然患者数量较少,但avalglucosidase alfa在日本患者中提供了疗效和安全性,代表了全球关键临床试验的总体人群。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy and safety of avalglucosidase alfa in Japanese patients with late-onset and infantile-onset Pompe diseases: A case series from clinical trials
Background
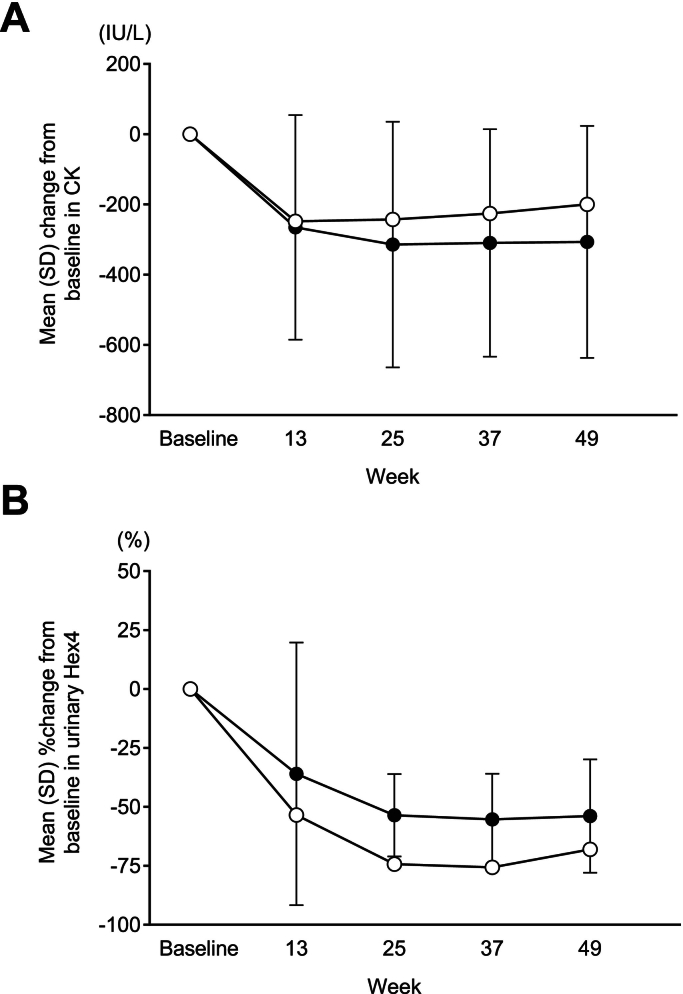
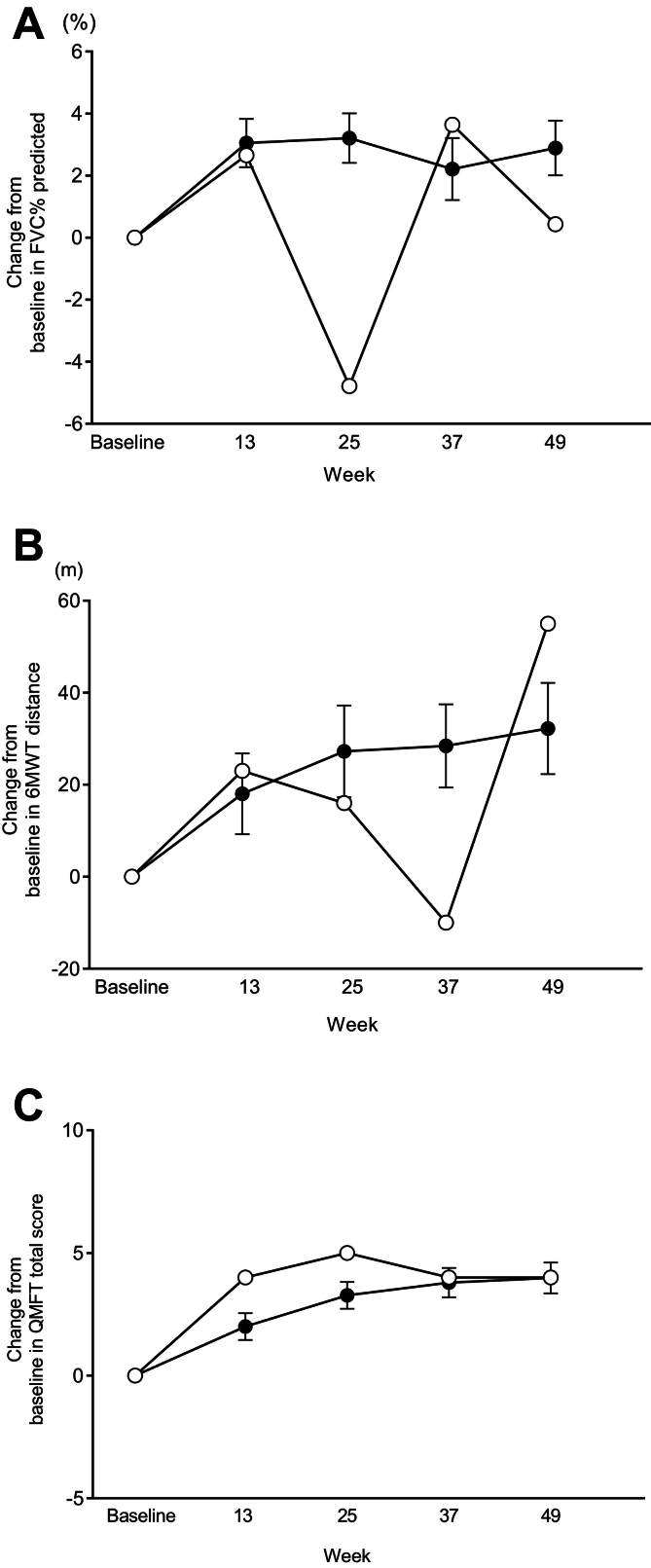
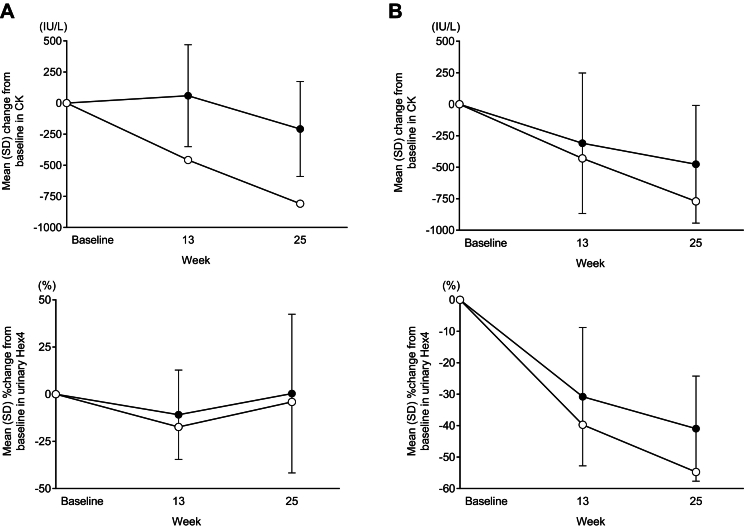
The efficacy and safety of avalglucosidase alfa for Pompe disease (PD) have been demonstrated in a global Phase 3 trial (COMET) in patients with late-onset PD (LOPD) and a global Phase 2 trial (Mini-COMET) in patients with infantile-onset PD (IOPD). This case series examines the individual results of three Japanese patients enrolled in these trials.
Methods
Case reports were assembled from data collected in the COMET and Mini-COMET trials. Detailed methods have been reported previously. The primary endpoint of COMET was change from baseline to week 49 in upright forced vital capacity percent (FVC %) predicted. The primary endpoint of Mini-COMET was safety and tolerability of avalglucosidase alfa. In both trials, key secondary endpoints included motor function tests and other qualitative measures of improvement. Changes in biomarkers and anti-drug antibodies were also assessed in both trials.
Results
Results for Japanese patients were representative of those from the overall populations in the COMET and Mini-COMET trials. We detail results for one Japanese patient with LOPD enrolled in the COMET trial and two Japanese patients with IOPD enrolled in the Mini-COMET trial. Importantly, avalglucosidase alfa was well tolerated at doses of both 20 mg/kg and 40 mg/kg in Japanese patients with LOPD and IOPD, respectively.
Conclusions
Although the number of patients was small, avalglucosidase alfa provides an efficacy and safety profile in Japanese patients representative of the overall populations from key global clinical trials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Genetics and Metabolism Reports
Biochemistry, Genetics and Molecular Biology-Endocrinology
CiteScore
4.00
自引率
5.30%
发文量
105
审稿时长
33 days
期刊介绍:
Molecular Genetics and Metabolism Reports is an open access journal that publishes molecular and metabolic reports describing investigations that use the tools of biochemistry and molecular biology for studies of normal and diseased states. In addition to original research articles, sequence reports, brief communication reports and letters to the editor are considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


