基于动态动力学分辨的外消旋2-取代喹啉不对称转移加氢反应
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
手性四氢喹啉(THQs)的合成由于其在生物活性化合物中经常作为药效团存在而引起了药物化学家的极大兴趣。虽然现有的合成方法主要集中于具有单个或多个内环手性中心的thq,但具有内环和外环手性中心的thq的选择性构建仍然是一个需要进一步发展的重大挑战。本研究介绍了一种基于动态动力学分辨率(DKR)的外消旋2-取代喹啉转移氢化反应,该反应产生具有连续内环和外环手性中心的结构新颖的手性thq,其产率和立体选择性都很好(59个例子,一般为20:1 dr和90% ee,最多三个连续的立体中心)。该方法为缺乏电子的芳香n-杂环的不对称转化提供了一种机制上的新方法,并为扩大药物化学的手性n-杂环化学空间提供了一种创新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
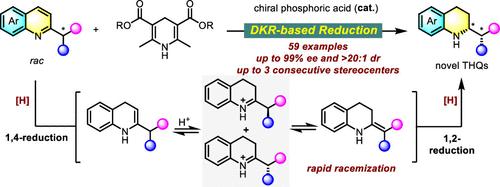
Dynamic Kinetic Resolution-Based Asymmetric Transfer Hydrogenation of Racemic 2-Substituted Quinolines
The synthesis of chiral tetrahydroquinolines (THQs) has garnered significant interest from medicinal chemists due to their frequent presence as pharmacophores in bioactive compounds. While existing synthetic methods have primarily focused on THQs with single or multiple endocyclic chiral centers, the selective construction of THQs with both endo- and exo-cyclic chiral centers remains a significant challenge that requires further development. This study introduces a dynamic kinetic resolution (DKR)-based transfer hydrogenation of racemic 2-substituted quinolines, which yields structurally novel chiral THQs with consecutive endo- and exo-cyclic chiral centers in excellent yields and stereoselectivities (59 examples, with generally >20:1 dr and >90% ee, up to three consecutive stereocenters). Our approach offers a mechanistically novel method for the asymmetric transformation of electron-deficient aromatic N-heterocycles and presents an innovative way to expand the chiral N-heterocycle chemical space for medicinal chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


