小型无客结构 II 水合物包埋异噁唑
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
包合物水合物作为一种多孔材料,在能量储存和转化方面具有很大的潜力,具有比现有水合物体系更安全、更环保的特点。在这里,我们报告了异恶唑,一个具有两个杂原子的五元分子,对各种笼形物水合物体系的影响,包括甲烷(CH4),二氧化碳(CO2)和乙烷(C2H6)小客体分子。我们证明了异恶唑分子与CH4和CO2形成结构II (sII)水合物,并作为结构I (C2H6)水合物的热力学抑制剂。有趣的是,我们的研究结果表明,异恶唑与C2H6在低压和低温条件下形成sII水合物。此外,与形成四氢呋喃和环戊烷水合物所需的温度相比,它可以在空的512笼中形成小的无客体sII水合物。我们的研究结果表明,sII(异恶唑)水合物在异恶唑和水骨架之间没有或非常弱的相互作用,不像四氢呋喃水合物暗示的主客体相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
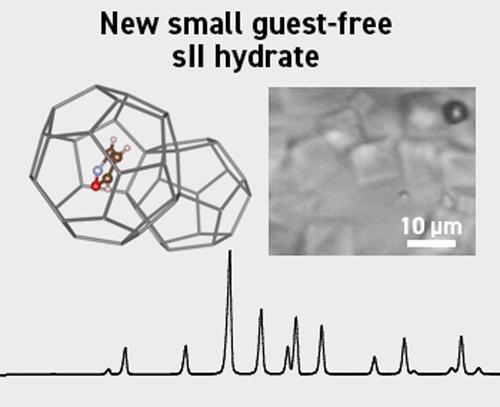
Small Guest-Free Structure II Hydrate Enclathrating Isoxazole
Clathrate hydrates hold promising potential as porous materials for energy storage and conversion, featuring safer and environmentally friendly alternatives to existing hydrate systems. Here, we report the impact of isoxazole, a five-membered molecule with two heteroatoms, on various clathrate hydrate systems, including methane (CH4), carbon dioxide (CO2), and ethane (C2H6) small guest molecules. We demonstrate that the isoxazole molecule forms structure II (sII) hydrates with CH4 and CO2 and acts as a thermodynamic inhibitor for structure I C2H6 hydrate. Interestingly, our results show that isoxazole forms an sII hydrate with C2H6 under low-pressure and low-temperature conditions. Moreover, it can form a small guest-free sII hydrate with a vacant 512 cage at much lower temperatures than those required for forming tetrahydrofuran and cyclopentane hydrates. Our findings demonstrate that sII (isoxazole) hydrate exhibits no or very weak interaction between isoxazole and the water framework, unlike tetrahydrofuran hydrate implying guest–host interaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


