硝酸电还原制氨高效TM/GaN单原子催化剂的理论筛选
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电催化硝酸(NO3 -)还原反应(NO3RR)是一种在温和条件下合成氨(NH3)的有前途的策略,为Haber-Bosch工艺提供了一种环保的替代方法。然而,现有的电化学NO3RR催化剂在选择性和催化剂稳定性方面存在局限性。本文利用密度泛函理论计算,研究了基于氮化镓(GaN)单层上掺杂过渡金属的新型高效单原子电化学还原硝酸盐为氨的选择性和活性催化剂。在18种候选催化剂中,V/GaN和Ru/GaN被认为是最有效的催化剂,分别具有-0.39 V和-0.46 V的低极限电位。从头算分子动力学模拟进一步证实了这些催化剂的动力学稳定性,在500k下,V/GaN和Ru/GaN的结构在5ps后保持稳定。我们的研究结果表明,V/GaN和Ru/GaN是很有前途的电催化剂,可以高效和选择性地将NO3RR转化为NH3,需要进一步的实验验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
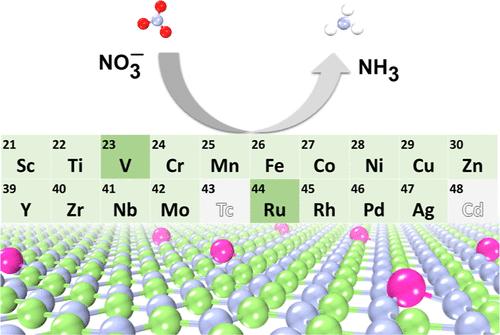
Theoretical Screening of Efficient TM/GaN Single-Atom Catalysts for Electroreduction of Nitrate to Ammonia
The electrocatalytic nitrate (NO3–) reduction reaction (NO3RR) represents a promising strategy for synthesizing ammonia (NH3) under milder conditions, providing an environmentally friendly alternative to the Haber–Bosch process. However, current electrochemical NO3RR catalysts are limited in terms of selectivity and catalyst stability. Herein, novel efficient single-atom catalysts for selective and active electrochemical reduction of nitrate to ammonia, based on transition metals doped on a gallium nitride (GaN) monolayer, are investigated by using density functional theory calculations. Among 18 candidates, V/GaN and Ru/GaN are identified as the most effective catalysts, demonstrating low limiting potentials of –0.39 V and –0.46 V, respectively. Ab initio molecular dynamics simulations further confirm the kinetic stability of these catalysts, with the structures of V/GaN and Ru/GaN remaining stable after 5 ps at 500 K. Our findings suggest that V/GaN and Ru/GaN are promising electrocatalysts for efficient and selective NO3RR to NH3, warranting further experimental validation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


