螺薄荷醇 A-D:云南濒危针叶树 Amentotaxus 中前所未有的 6/6/6/5/6/6/6 螺八环双二萜杂二聚体及其生物活性
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-01-22
DOI:10.1039/d4qo02210f
引用次数: 0
摘要
作为一种新的双二萜杂二聚体化学类别,螺烷三醇 A-D (1-4) 的特点是具有复杂的 6/6/6/5/6/6/6 螺辛环系统,其生物来源可能是烯烃和 C20-norabietane 之间的 Diels-Alder [4 + 2] 环加成。螺根他克酚具有独特的螺[双环[3.2.1]辛烷-7,2′-双环[2.2.2]辛烯]结构。通过分子离子网络(MoIN)的应用,这些分子间 Diels-Alder 异构体从中国濒危针叶树 Amentotaxus yunnanensis 的可再生枝条和针叶中分离出来。利用光谱方法、电子圆二色性计算和 X 射线衍射分析阐明了它们的化学结构。从结构多样性的角度出发,从相对主要的化合物 1 合成了两种半合成类似物,即 7′-脱氧-6′-烯螺烷紫杉醇 A(1a)和 7′-脱氧-螺烷紫杉醇 A(1b)。其中,化合物 1 在 20 μM 的无毒浓度下可减轻 RAW 264.7 巨噬细胞和 BV2 微神经胶质细胞的炎症反应,对脂多糖(LPS)诱导的一氧化氮产生的抑制率分别为 46.66% 和 32.37%。1b 对一组人类癌细胞株(A549、MCF7、HCT116、RKO 和 HepG2)具有疗效,IC50 值介于 6.27 到 14.10 μM 之间。在 HCT116 细胞中,1b 特别影响 G2 期细胞周期的进展并诱导细胞凋亡。这些发现强调了保护植物物种多样性的重要性,它是维持化学多样性的一种手段,也是治疗癌症的新疗法的潜在来源。本文章由计算机程序翻译,如有差异,请以英文原文为准。

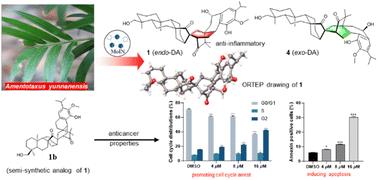
Spiroamentotaxols A–D: unprecedented 6/6/6/5/6/6/6/6 spiro-octacyclic bis-diterpene heterodimers from the endangered conifer Amentotaxus yunnanensis and their bioactivities†
As a new chemical class of bis-diterpene heterodimers, spiroamentotaxols A–D () are characterized by a complex 6/6/6/5/6/6/6/6 spiro-octacyclic ring system, which is likely biogenetically derived from a Diels–Alder [4 + 2] cycloaddition between an ent-kaurene and a C20-norabietane. The spiroamentotaxols possess a distinctive spiro[bicyclo[3.2.1]octane-7,2′-bicyclo[2.2.2]octene] motif. Through the application of molecular ion networking (MoIN), these intermolecular Diels–Alder isomers were isolated from the renewable twigs and needles of the endangered Chinese conifer Amentotaxus yunnanensis. Their chemical structures were elucidated using spectroscopic methods, electronic circular dichroism calculations, and X-ray diffraction analysis. From a structural diversity perspective, two semi-synthetic analogs, namely, 7′-deoxy-6′-en-spiroamentotaxol A () and 7′-deoxy-spiroamentotaxol A (), were synthesized from the relatively major compound . These spiro-polycyclic compounds were evaluated for their in vitro anti-inflammatory and anticancer activities. In particular, compound attenuated inflammation in both RAW 264.7 macrophage and BV2 microglial cells at a non-toxic concentration of 20 μM, with the lipopolysaccharide (LPS)-induced nitric oxide production inhibition rates of 46.66% and 32.37%, respectively. exhibited efficacy against a panel of human cancer cell lines (A549, MCF7, HCT116, RKO, and HepG2), with IC50 values ranging from 6.27 to 14.10 μM. In the case of HCT116 cells, specifically influenced cell-cycle progression at the G2 phase and induced apoptosis. The findings highlight the importance of conserving plant species diversity as a means to sustain chemical diversity and serve as a potential source of new therapeutic agents for cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


